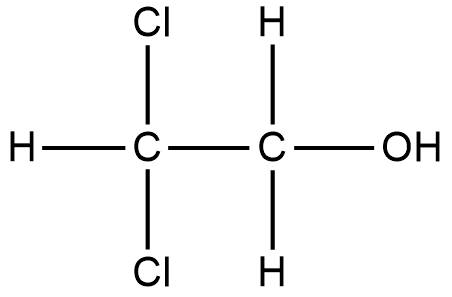
What kind of reaction is CH4 Cl2?
substitution reactionThis reaction is an example of substitution reaction.
How do you balance CH4 Cl2?
0:012:12How to Balance CH4 + Cl2 = HCl + CHCl3 (Methane + Chlorine gas)YouTubeStart of suggested clipEnd of suggested clipUntil last we have cl2 by itself it's only chlorine atoms so when we change this it won't change theMoreUntil last we have cl2 by itself it's only chlorine atoms so when we change this it won't change the number of carbons or hydrogens. So we'll start balancing the hydrogens.
Is CH4 Cl2 a substitution reaction?
CH4+Cl2→CH2Cl2+HCl. Hint: Substitution reaction is a reaction in which one atom of a molecule is replaced with an atom of another molecule. When methane reacts with chlorine in presence of diffused sunlight to produce compounds containing chlorine then this reaction is called chlorination of methane.
Is this equation balanced or unbalanced CH4 Cl2 → CCl4 HCl?
0:231:35How to Balance CH4 + Cl2 = CCl4 + HCl (Methane + Chlorine gas)YouTubeStart of suggested clipEnd of suggested clipSo this equation is balanced.MoreSo this equation is balanced.
How do you balance equations?
10:0520:52Introduction to Balancing Chemical Equations - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo what we need to do is multiply the equation. By two. So two so2 two times a half we said it's oneMoreSo what we need to do is multiply the equation. By two. So two so2 two times a half we said it's one so one o2 produces two so3 molecules notice that it's balanced at this point.
What is methane a compound of?
MethaneMethane / IUPAC IDMethane (US: MEH-thayn, UK: MEE-thayn) is a chemical compound with the chemical formula CH4 (one carbon atom bonded to four hydrogen atoms). It is a group-14 hydride, the simplest alkane, and the main constituent of natural gas.
Why is the reaction between methane and chlorine called the substitution reaction?
One of the hydrogen atoms in the methane has been replaced by a chlorine atom, so this is a substitution reaction. However, the reaction does not stop there, and all the hydrogens in the methane can in turn be replaced by chlorine atoms.
What type of reaction is free radical substitution?
A free radical substitution reaction is one involving these radicals. Free radicals are formed if a bond splits evenly - each atom getting one of the two electrons. The name given to this is homolytic fission....Free radical reactions.a chlorine radicalCla methyl radicalCH3Sep 12, 2020
What is free radical chain reaction?
A free-radical reaction is a chemical mechanism in which unpaired electrons have molecules involved. The radical species may be a starting compound or a product, but the most common cases are reactions involving radicals as intermediates in organic chemistry.
What type of reaction is ch4 cl2 CCl4 HCL?
Methane reacts with chlorine gas to form an important compound carbon tetrachloride along with hydrochloric acid. In this reaction, one mole of methane gas reacts with four moles of chlorine gas to form one mole of carbon tetrachloride along with four moles of hydrochloric acid.
Why do we balance chemical equations?
A chemical equation needs to be balanced so as to make the number of the atoms of the reactants equal to the number of the atoms of the products.
What does the reagent CCl4 do?
It was used in the production of refrigeration fluid and propellants for aerosol cans, as a pesticide, as a cleaning fluid and degreasing agent, in fire extinguishers, and in spot removers. Because of its harmful effects, these uses are now banned and it is only used in some industrial applications.
How do you balance CH4?
0:422:15Balanced Equation for the Combustion of Methane (CH4)YouTubeStart of suggested clipEnd of suggested clipSo to balance the equation where we're burning the methane here in oxygen let's count the atoms upMoreSo to balance the equation where we're burning the methane here in oxygen let's count the atoms up on each side we have one carbon four hydrogens.
How do you balance the equation c10h16 cl2 C HCl?
0:031:23How to Balance C10H16 + Cl2 = C + HCl - YouTubeYouTubeStart of suggested clipEnd of suggested clip1 times 16 that equals 16 we've balanced the hydrogens. And then 1 times 16 that'll give us 16More1 times 16 that equals 16 we've balanced the hydrogens. And then 1 times 16 that'll give us 16 chlorine atoms kind of easy to fix that though we could just put an 8 in front of the cl2.
Is this equation balanced or unbalanced Fe cl2 ⟶ fecl3?
0:011:46Balancing the Equation Fe + Cl2 = FeCl3 (and Type of Reaction)YouTubeStart of suggested clipEnd of suggested clipYou could also call this a redox reaction because the iron and the chlorine have changed theirMoreYou could also call this a redox reaction because the iron and the chlorine have changed their oxidation states let's balance the equation. In the reactants.
What is the balanced equation for c4h10 o2?
0:012:41Balancing C4H10 + O2 = CO2 + H2O (Butane + Oxygen gas) - YouTubeYouTubeStart of suggested clipEnd of suggested clipFour carbons 10 hydrogen's. And two oxygen atoms on the product side we have the one carbon twoMoreFour carbons 10 hydrogen's. And two oxygen atoms on the product side we have the one carbon two hydrogen's. And then two oxygens.
Phenomenon
This equation does not have any specific information about phenomenon.
Tham gia thảo luận
This website uses cookies to ensure you get the best experience on our website.
chemistry
Hydrogen and chlorine react to yield hydrogen chloride: H2+ Cl2 ( 2HCl. How many grams of HCl are formed from reaction of 3.56 g of H2 with 8.94 g of Cl2? Which reactant is limiting?
Chemistry
1.) The the following reaction is: BaCO3 --> BaO + CO2 Decomposition reaction Single replacement reaction Combustion Reaction Synthesis Reaction Double replacement reaction 2.) When magnesium is burned in the presence of oxygen,
science chemistry
Given 4K + O2 → 2K2O, what is the reaction type? Single Replacement Double Replacement Synthesis (formation) Decomposition
chemistry
Which of the following equations correctly describes the relationship between the rate at which NO2 and Cl2 are consumed in the following reaction? 2 NO2 (g) + Cl2 (g) → 2 NO2Cl (g) A. -d (NO2)/dt = 1/2 [d (Cl2)/dt] B. -d (NO2)/dt = 2
Chemistry
The equation Ba (s) + HCl (aq)→BaCl2 (aq) + H2 (g) is an example of which type of reaction? A. double-replacement B. combustion C. single-replacement D. decomposition C?
Chemistry
A mixture of 0.47 mole of H2 and 3.59 moles of HCl is heated to 2800C. Calculate the equilibrium partial pressures of H2 Cl2 and HCl if the total pressure is 2.00 atm. For the reaction Kp is 193 at 2800C. H2 (g) +Cl2 (g) = 2HCl (g)
chemistry
a. Cl2 + 2NaI → 2NaCl + I2 This is a single replacement reaction. b. 2K + 2HCl → 2KCl + H2 This is a single replacement reaction. c. N2 + 3H2 → 2NH3 This is a synthesis reaction. d. 2KClO3 → 2KCl + 3O2 This is a
