
What is the Order of the electron transport chain?
- The cytochromes are conjugated proteins containing heme
- The iron of heme in cytochromes is alternately oxidized (Fe 3+ ) and reduced (Fe 2+ ), which is essential for the transport of electrons in the ETC.
- The electrons are transported from coenzyme Q to cytochromes (in the order) b, c 1 , c 2 , a and a 3.
What are the main products of the electron transport chain?
What are the products and reactants of the electron transport chain?
- Reactants and products of ETC. Electron Transport reactants: Hydrogen ions, oxygen, NADH, FADH2 Products:Water and ATP ( 2 e- + 2 H+ 1/2 O2= H20)
- Complex I. NADH dehydrogenase.
- Complex II.
- Complex III.
- Complex IV.
- Role of Oxygen in ETC.
- Substrate Level Phosphorylation.
- Oxidative Phosphorylation.
What are the steps of electron transport?
The three main steps in the electron transport chain are:
- Generation of a proton gradient across the mitochondrial membrane. Proton accumulation occurs in the intermembrane space of mitochondria.
- Reduction of molecular oxygen and formation of water.
- ATP synthesis by chemiosmosis.
What is the process of electron transport chain?
Things to Remember
- In eukaryotes, mitochondria is the site for aerobic respiration. ...
- The electron transport chain consists of 5 complexes present in a sequence that carry out the transfer of electrons and coupled ATP production.
- Electrons in the electron transport chain are transferred from molecules of lower redox potential to that of higher redox potential.

What is the name of complex 4 in electron transport chain?
cytochrome c oxidaseComplex IV is also known as cytochrome c oxidase. It contains cytochrome a and a3, two heme and two copper centres. Complex IV receives electrons from cytochrome c, hence the name cytochrome c oxidase. Complex IV transfers electrons to O2.
What happens in complex 4 of electron transport chain?
Complex IV of the electron transport chain, also known as cytochrome c oxidase, is a multiunit structure that functions to transfer electrons form cytochrome c to oxygen and in the process form water and help generate a proton gradient.
Why is complex IV important?
Cytochrome c oxidase or complex IV, catalyzes the final step in mitochondrial electron transfer chain, and is regarded as one of the major regulation sites for oxidative phosphorylation. This enzyme is controlled by both nuclear and mitochondrial genomes.
What inhibits complex 4 of the electron transport chain?
CyanideCyanide: inhibits terminal electron transfer to oxygen, Complex IV.
What happens when complex 4 is inhibited?
The blocklock of complex IV by cyanide depletes ATP culminating in cell death. Oxygen is unable to reoxidize the reduced cytochrome a3. Thus, cellular respiration is inhibited, as well as ATP production, in essence depriving the cells, tissue, and, ultimately, the whole body of oxygen.
Which of the following best describes the role of complex IV in the electron transport chain?
Which of the following best describes complex IV in the electron transport chain? a. Complex IV consists of an oxygen molecule held between the cytochrome and copper ions. The electrons flowing finally reach the oxygen, producing water.
Is cytochrome c part of complex IV?
It is the terminal oxidase of the respiratory chain in the transfer of electrons from cyt c to O2. Cytochrome c is not an integral part of complex IV, but is stoichiometrically associated with it and is believed to be spatially associated with subunit II of cytochrome oxidase.
What components of the electron transport pathway are associated with complex IV?
Complex IV The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the cytochromes a and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3).
What are the types of cytochrome present in complex IV?
Complex IV contains a cytochrome a/a3-domain that transfers electrons and catalyzes the reaction of oxygen to water. Photosystem II, the first protein complex in the light-dependent reactions of oxygenic photosynthesis, contains a cytochrome b subunit.
How many protons does complex IV pump?
two protonsIn complex IV, two protons per pair of electrons are pumped across the membrane and another two protons per pair of electrons are combined with O2 to form H2O within the matrix.
What are the complexes in electron transport chain?
The ETC proteins in a general order are complex I, complex II, coenzyme Q, complex III, cytochrome C, and complex IV.
Which of the four complexes pump protons out of the mitochondrial matrix?
Which of the four complexes pump protons out of the mitochondrial matrix? All four complexes pump protons out of the mitochondrial matrix.
What is the fate of the 4 electrons from cytochrome c in complex IV of the respiratory pathway?
Four electrons are used to reduce one oxygen molecule into two water molecules.
Which of the four complexes pump protons out of the mitochondrial matrix?
Which of the four complexes pump protons out of the mitochondrial matrix? All four complexes pump protons out of the mitochondrial matrix.
How many protons does complex IV pump out?
two protonsIn complex IV, two protons per pair of electrons are pumped across the membrane and another two protons per pair of electrons are combined with O2 to form H2O within the matrix. Thus, the equivalent of four protons per pair of electrons are transported out of the mitochondrial matrix at each of these three complexes.
Where are protein complexes I through IV of the electron transport chain located?
Where are protein complexes I'll through IV of the electron transport chain located? NADH and FADH2 are electron donors mitochondrial electron transport chain. NADPH Is the electron donor for the Calvin cycle, which takes place in the chloroplast stroma.
How does FADH 2 work?
This enzyme and FADH 2 form a small complex that delivers electrons directly to the electron transport chain, bypassing the first complex. Since these electrons bypass and thus do not energize the proton pump in the first complex, fewer ATP molecules are made from the FADH 2 electrons. The number of ATP molecules ultimately obtained is directly ...
How is ATP generated during aerobic catabolism?
Rather, it is derived from a process that begins with moving electrons through a series of electron transporters that undergo redox reactions: the electron transport chain.
Why is DNP poisoning so effective?
Show Answer. After DNP poisoning, the electron transport chain can no longer form a proton gradient, and ATP synthase can no longer make ATP. DNP is an effective diet drug because it uncouples ATP synthesis ; in other words, after taking it, a person obtains less energy out of the food he or she eats.
What is the process of electron transport?
Electron transport is a series of redox reactions that resemble a relay race or bucket brigade in that electrons are passed rapidly from one component to the next, to the endpoint of the chain where the electrons reduce molecular oxygen, producing water.
How does a concentration gradient form?
Therefore, a concentration gradient forms in which hydrogen ions diffuse out of the matrix space by passing through ATP synthase. The current of hydrogen ions powers the catalytic action of ATP synthase, which phosphorylates ADP, producing ATP. Figure 1.
What are the pathways of cellular respiration?
You have just read about two pathways in cellular respiration—glycolysis and the citric acid cycle —that generate ATP. However, most of the ATP generated during the aerobic catabolism of glucose is not generated directly ...
How do hydrogen ions diffuse?
If the membrane were open to diffusion by the hydrogen ions, the ions would tend to diffuse back across into the matrix, driven by their electrochemical gradient. Recall that many ions cannot diffuse through the nonpolar regions of phospholipid membranes without the aid of ion channels. Similarly, hydrogen ions in the matrix space can only pass through the inner mitochondrial membrane through an integral membrane protein called ATP synthase (Figure 2). This complex protein acts as a tiny generator, turned by the force of the hydrogen ions diffusing through it, down their electrochemical gradient. The turning of parts of this molecular machine facilitates the addition of a phosphate to ADP, forming ATP, using the potential energy of the hydrogen ion gradient.
How does the electron transport chain work?
Energy obtained through the transfer of electrons down the electron transport chain is used to pump protons from the mitochondrial matrix into the intermembrane space , creating an electrochemical proton gradient ( ΔpH) across the inner mitochondrial membrane. This proton gradient is largely but not exclusively responsible for the mitochondrial membrane potential (ΔΨ M ). It allows ATP synthase to use the flow of H + through the enzyme back into the matrix to generate ATP from adenosine diphosphate (ADP) and inorganic phosphate. Complex I (NADH coenzyme Q reductase; labeled I) accepts electrons from the Krebs cycle electron carrier nicotinamide adenine dinucleotide (NADH), and passes them to coenzyme Q (ubiquinone; labeled Q), which also receives electrons from complex II ( succinate dehydrogenase; labeled II). Q passes electrons to complex III ( cytochrome bc 1 complex; labeled III), which passes them to cytochrome c (cyt c ). Cyt c passes electrons to complex IV ( cytochrome c oxidase; labeled IV), which uses the electrons and hydrogen ions to reduce molecular oxygen to water.
What is the chain of electron transport?
The electron transport chain ( ETC; respiratory chain) is a series of protein complexes that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H + ions) across a membrane. The electron transport chain is built up of peptides, enzymes, and other molecules.
Why is the electron transport chain in bacteria more complicated?
In prokaryotes ( bacteria and archaea) the situation is more complicated, because there are several different electron donors and several different electron acceptors. The generalized electron transport chain in bacteria is:
Which electron donor directs electrons into Q?
Other electron donors (e.g., fatty acids and glycerol 3-phosphate) also direct electrons into Q (via FAD). Complex II is a parallel electron transport pathway to complex 1, but unlike complex 1, no protons are transported to the intermembrane space in this pathway.
What happens to electrons when they are passed to oxygen?
Each electron donor will pass electrons to a more electronegative acceptor, which in turn donates these electrons to another acceptor, a process that continues down the series until electrons are passed to oxygen, the most electronegative and terminal electron acceptor in the chain.
What is the flow of electrons in the electron transport chain?
The flow of electrons through the electron transport chain is an exergonic process. The energy from the redox reactions create an electrochemical proton gradient that drives the synthesis of adenosine triphosphate (ATP). In aerobic respiration, the flow of electrons terminates with molecular oxygen being the final electron acceptor.
Why is oxygen used as an electron acceptor?
In aerobic bacteria and facultative anaerobes if oxygen is available, it is invariably used as the terminal electron acceptor, because it generates the greatest Gibbs free energy change and produces the most energy.
What is antimycin A?
Antimycin A is a piscicide that binds to cytochrome c reductase at the Qi binding site. This activity prevents ubiquinone from binding and accepting an electron, thereby blocking the recycling of ubiquinol (CoQH2) by the Q cycle.
What is complex 3?
Complex III, also known as cytochrome c reductase, is made up of cytochrome b, Rieske subunits (containing two Fe-S clusters), and cytochrome c proteins. A cytochrome is a protein involved in electron transfer that contains a heme group. The heme groups alternate between ferrous (Fe2+) and ferric (Fe3+) states during the electron transfer. Because cytochrome c can only accept a single electron at a time, this process occurs in two steps (the Q cycle), in contrast to the single-step complex I and II pathways. Complex III also releases 4 protons into the intermembrane space at the end of a full Q cycle, contributing to the gradient. Cytochrome c then transfers the electrons one at a time to complex IV. [9][10][11]
What is the first step in the Q cycle?
Step 1 in the Q cycle involves ubiquinol (CoQH2) and ubiquinone (CoQ) binding to two separate sites on complex III. CoQH2 transfers each electron to a different path. One electron goes to Fe-S and then cytochrome c, while the second electron is transferred to cytochrome b and then to CoQ bound at the other site. While this occurs, 2 H+ ions are released into the intermembrane space, contributing to the proton gradient. CoQH2 is now oxidized to ubiquinone and dissociates from the complex. The CoQ bound at the second site enters a transitional CoQH- radical state from accepting one of the electrons.
What is the role of complex IV in cellular respiration?
Complex IV, also known as cytochrome c oxidase, oxidizes cytochrome c and transfers the electrons to oxygen, the final electron carrier in aerobic cellular respiration . The cytochrome proteins a and a3, in addition to heme and copper groups in complex IV transfer the donated electrons to the bound dioxygen species, converting it into molecules of water. The free energy from the electron transfer causes 4 protons to move into the intermembrane space contributing to the proton gradient. Oxygen reduces via the following reaction:[13][14]
What are the parts of aerobic respiration?
Aerobic cellular respiration is made up of three parts: glycolysis, the citric acid (Krebs) cycle, and oxidative phosphorylation. In glycolysis, glucose metabolizes into two molecules of pyruvate, with an output of ATP and nicotinamide adenine dinucleotide (NADH). Each pyruvate oxidizes into acetyl CoA and an additional molecule of NADH and carbon dioxide (CO2). The acetyl CoA is then used in the citric acid cycle, which is a chain of chemical reactions that produce CO2, NADH, flavin adenine dinucleotide (FADH2), and ATP. In the final step, the three NADH and one FADH2 amassed from the previous steps are used in oxidative phosphorylation, to make water and ATP.
How does the electron transport chain work?
In the electron transport chain (ETC), the electrons go through a chain of proteins that increases its reduction potential and causes a release in energy. Most of this energy is dissipated as heat or utilized to pump hydrogen ions (H+) from the mitochondrial matrix to the intermembrane space and create a proton gradient. This gradient increases the acidity in the intermembrane space and creates an electrical difference with a positive charge outside and a negative charge inside. The ETC proteins in a general order are complex I, complex II, coenzyme Q, complex III, cytochrome C, and complex IV.
What is the second step of the CoQH2 cycle?
The second step of the cycle involves a repeat of the first: a new CoQH2 binds to the first site and transfers two electrons like before (and 2 more H+ ions released). Again, one electron passes to cytochrome c and one to cytochrome b, which this time works to reduce CoQH- to CoQH2 before it dissociates from complex III and can be recycled. In this way, one full cycle appears as follows:[12]
Where Does the Electron Transport Chain Occur?
During the process, a proton gradient is created when the protons are pumped from the mitochondrial matrix into the intermembrane space of the cell, which also helps in driving ATP production. Often, the use of a proton gradient is referred to as the chemiosmotic mechanism that drives ATP synthesis since it relies on a higher concentration of protons to generate “proton motive force”. The amount of ATP created is directly proportional to the number of protons that are pumped across the inner mitochondrial membrane.
How many electrons does NADH have?
The NADH now has two electrons passing them onto a more mobile molecule, ubiquinone (Q), in the first protein complex (Complex I). Complex I, also known as NADH dehydrogenase, pumps four hydrogen ions from the matrix into the intermembrane space, establishing the proton gradient.
What is the ATP synthase?
As the proton gradient is established, F 1 F 0 ATP synthase, sometimes referred to as Complex V, generates the ATP. The complex is composed of several subunits that bind to the protons released in prior reactions. As the protein rotates, protons are brought back into the mitochondrial matrix, allowing ADP to bind to free phosphate to produce ATP. For every full turn of the protein, three ATP is produced, concluding the electron transport chain.
How do electrons move in the electron transfer chain?
In the electron transfer chain, electrons move along a series of proteins to generate an expulsion type force to move hydrogen ions, or protons, across the mitochondrial membrane. The electrons begin their reactions in Complex I, continuing onto Complex II, traversed to Complex III and cytochrome c via coenzyme Q, and then finally to Complex IV. The complexes themselves are complex-structured proteins embedded in the phospholipid membrane. They are combined with a metal ion, such as iron, to help with proton expulsion into the intermembrane space as well as other functions. The complexes also undergo conformational changes to allow openings for the transmembrane movement of protons.
What is the Q cycle?
Complex III, or cytochrome c reductase, is where the Q cycle takes place. There is an interaction between Q and cytochromes, which are molecules composed of iron , to continue the transfer of electrons. During the Q cycle, the ubiquinol (QH 2) previously produced donates electrons to ISP and cytochrome b becoming ubiquinone. ISP and cytochrome b are proteins that are located in the matrix that then transfers the electron it received from ubiquinol to cytochrome c1. Cytochrome c1 then transfers it to cytochrome c, which moves the electrons to the last complex. (Note: Unlike ubiquinone (Q), cytochrome c can only carry one electron at a time). Ubiquinone then gets reduced again to QH 2, restarting the cycle. In the process, another hydrogen ion is released into the cytosol to further create the proton gradient.
How is ATP generated in an exothermic reaction?
energy is released in an exothermic reaction when electrons are passed through the complexes ; three molecules of ATP are created. Phosphate located in the matrix is imported via the proton gradient, which is used to create more ATP. The process of generating more ATP via the phosphorylation of ADP is referred to oxidative phosphorylation since the energy of hydrogen oxygenation is used throughout the electron transport chain. The ATP generated from this reaction go on to power most cellular reactions necessary for life.
What is the mechanism that drives ATP synthesis?
Often, the use of a proton gradient is referred to as the chemiosmotic mechanism that drives ATP synthesis since it relies on a higher concentration of protons to generate “proton motive force”. The amount of ATP created is directly proportional to the number of protons that are pumped across the inner mitochondrial membrane. ...
What is the role of cardiolipin in holoenzyme?
Both dimers are connected by a cardiolipin molecule, which has been found to play a key role in stabilization of the holoenzyme complex. The dissociation of subunits VIIa and III in conjunction with the removal of cardiolipin results in total loss of enzyme activity.
What are the three states of COX?
COX exists in three conformational states: fully oxidized (pulsed), partially reduced, and fully reduced. Each inhibitor has a high affinity to a different state. In the pulsed state, both the heme a 3 and the Cu B nuclear centers are oxidized; this is the conformation of the enzyme that has the highest activity. A two-electron reduction initiates a conformational change that allows oxygen to bind at the active site to the partially-reduced enzyme. Four electrons bind to COX to fully reduce the enzyme. Its fully reduced state, which consists of a reduced Fe 2+ at the cytochrome a 3 heme group and a reduced Cu B+ binuclear center, is considered the inactive or resting state of the enzyme.
How many subunits does cytochrome C oxidase have?
Cytochrome c oxidase has 3 subunits which are encoded by mitochondrial DNA (cytochrome c oxidase subunit I, subunit II, and subunit III ). Of these 3 subunits encoded by mitochondrial DNA, two have been identified in extramitochondrial locations.
What is the ligand that is protonated and lost as water?
The hydroxide ligand is protonated and lost as water, creating a void between the metals that is filled by O 2. The oxygen is rapidly reduced, with two electrons coming from the Fe 2+ cytochrome a 3, which is converted to the ferryl oxo form (Fe 4+ =O).
How many proteins are in a complex?
The complex. The complex is a large integral membrane protein composed of several metal prosthetic sites and 14 protein subunits in mammals. In mammals, eleven subunits are nuclear in origin, and three are synthesized in the mitochondria. The complex contains two hemes, a cytochrome a and cytochrome a 3, and two copper centers, ...
What are the two hemes in the cytochrome complex?
The complex contains two hemes, a cytochrome a and cytochrome a 3, and two copper centers, the Cu A and Cu B centers. In fact, the cytochrome a 3 and Cu B form a binuclear center that is the site of oxygen reduction.
Where does cytochrome C dock?
Cytochrome c, which is reduced by the preceding component of the respiratory chain (cytochrome bc1 complex, complex III), docks near the Cu A binuclear center and passes an electron to it, being oxidized back to cytochrome c containing Fe 3+.
What is the role of complex II in the citric acid cycle?
These come from FADH2, from the citric acid cycle. Complex II relieves FADH2 of its electrons, and passes them to CoQ . The coenzyme passes them to complex III, which now receives electrons and their energy from two sources. This allows complex III to pump large amounts of hydrogen across the membrane. Cytochrome c (Cyt c) allows the electrons to be passed to complex IV, the final complex in the electron transport chain. This complex passes the electrons to oxygen molecules, where they bind with hydrogens to produce water. With the final bit of energy, another proton is passed through the membrane.
How is photophosphorylation similar to oxidative phosphorylation?
Interestingly, the process of photophosphorylation is very similar to oxidative phosphorylation. This process is used in photosynthesis. However, instead of using oxygen to create water, it uses water to create oxygen. Basically the opposite of oxidative phosphorylation, photosynthesis uses an electron transport chain of its own to carry energy from sunlight into the bonds of sugar molecules. The plant can then use these molecules to feed other cells within its body. Just as an animal would, it breaks the glucose into pyruvate, and the pyruvate enters the mitochondria and eventually undergoes oxidative phosphorylation powered by the electron transport chain.
What are the two types of coenzymes that are involved in the electron transport chain?
The most common coenzymes are nicotinamide adenine dinucleotide (NAD) and flavin adenine dinucleotide (FA D). NAD can be reduced with electrons and a proton to become NADH, while FAD can take on two protons and four electrons to become FADH2. These coenzymes can bind to the proteins of the electron transport chain, and transfer their electrons and protons. This becomes the first stage in the electron transport chain.
What is oxidative phosphorylation?
Oxidative phosphorylation is a process involving a flow of electrons through the electron transport chain, a series of proteins and electron carriers within the mitochondrial membrane. This flow of electrons allows the electron transport chain to pump protons to one side of the mitochondrial membrane. As the protons build up, they create ...
How many proteins are in the electron transport chain?
The electron transport chain consists of four protein complexes, simply named complex I, complex II, complex III, and complex IV. Each complex is designed to receive electrons from a coenzyme or one of the other complexes in the chain. The actions each complex takes can be seen in the image below.
What does the yellow line in the photo represent?
The yellow lines in the image represent the generation of reduced coenzymes, or molecules which are carrying electrons. While some ATP is generated during glycolysis and the citric acid cycle, the majority is generated through oxidative phosphorylation. The electron transport chain is symbolized by the red staircase, ...
What are the steps of cellular respiration?
Oxidative phosphorylation is part of a larger system, cellular respiration. The 4 steps of cellular respiration can be seen in the image below. The first step occurs outside of the mitochondria. This involves the breakdown of glucose, lipids, or amino acids. This step is symbolized here with “Glycolysis” only. Remember that there are other ways to generate pyruvate and intermediates the Krebs cycle (citric acid cycle).
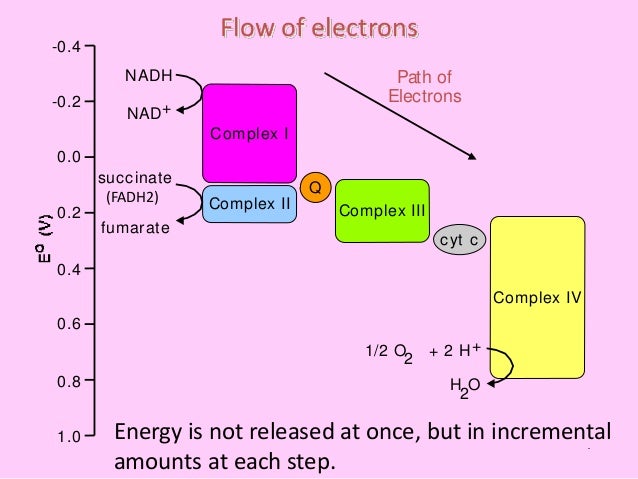
Complex I
Q and Complex II
- Complex II directly receives FADH2, which does not pass through complex I. The compound connecting the first and second complexes to the third is ubiquinone (Q). The Q molecule is lipid soluble and freely moves through the hydrophobic core of the membrane. Once it is reduced, (QH2), ubiquinone delivers its electrons to the next complex in the electron transport chain. Q re…
Complex III
- The third complex is composed of cytochrome b, another Fe-S protein, Rieske center (2Fe-2S center), and cytochrome c proteins; this complex is also called cytochrome oxidoreductase. Cytochrome proteins have a prosthetic group of heme. The heme molecule is similar to the heme in hemoglobin, but it carries electrons, not oxygen. As a result, the iron ion at its core is reduced …
Complex IV
- The fourth complex is composed of cytochrome proteins c, a, and a3. This complex contains two heme groups (one in each of the two cytochromes, a, and a3) and three copper ions (a pair of CuA and one CuB in cytochrome a3). The cytochromes hold an oxygen molecule very tightly between the iron and copper ions until the oxygen is completely reduced. Th...
Chemiosmosis
- In chemiosmosis, the free energy from the series of redox reactions just described is used to pump hydrogen ions (protons) across the membrane. The uneven distribution of H+ions across the membrane establishes both concentration and electrical gradients (thus, an electrochemical gradient), owing to the hydrogen ions’ positive charge and their aggregation on one side of the m…
ATP Yield
- The number of ATP molecules generated from the catabolism of glucose varies. For example, the number of hydrogen ions that the electron transport chain complexes can pump through the membrane varies between species. Another source of variance stems from the shuttle of electrons across the membranes of the mitochondria. (The NADH generated from glycolysis ca…
Overview
An electron transport chain (ETC ) is a series of protein complexes and other molecules that transfer electrons from electron donors to electron acceptors via redox reactions (both reduction and oxidation occurring simultaneously) and couples this electron transfer with the transfer of protons (H ions) across a membrane.A series of proteins in the inner membrane of mitochondria. The el…
Mitochondrial electron transport chains
Most eukaryotic cells have mitochondria, which produce ATP from reactions of oxygen with products of the citric acid cycle, fatty acid metabolism, and amino acid metabolism. At the inner mitochondrial membrane, electrons from NADH and FADH2 pass through the electron transport chain to oxygen, which provides the energy driving the process as it is reduced to water. The electron transpor…
Bacterial electron transport chains
In eukaryotes, NADH is the most important electron donor. The associated electron transport chain is NADH → Complex I → Q → Complex III → cytochrome c → Complex IV → O2 where Complexes I, III and IV are proton pumps, while Q and cytochrome c are mobile electron carriers. The electron acceptor for this process is molecular oxygen.
In prokaryotes (bacteria and archaea) the situation is more complicated, because there are severa…
Photosynthetic
In oxidative phosphorylation, electrons are transferred from an electron donor such as NADH to an acceptor such as O2 through an electron transport chain, releasing energy. In photophosphorylation, the energy of sunlight is used to create a high-energy electron donor which can subsequently reduce oxidized components and couple to ATP synthesis via proton translocation by the electron transport chain.
See also
• Charge-transfer complex
• CoRR hypothesis
• Electron equivalent
• Hydrogen hypothesis
• Respirasome
Further reading
• Fenchel T, King GM, Blackburn TH (September 2006). Bacterial Biogeochemistry: The Ecophysiology of Mineral Cycling (2nd ed.). Elsevier. ISBN 978-0-12-103455-9.
• Lengeler JW (January 1999). Drews G; Schlegel HG (eds.). Biology of the Prokaryotes. Blackwell Science. ISBN 978-0-632-05357-5.
External links
• Electron+Transport+Chain+Complex+Proteins at the US National Library of Medicine Medical Subject Headings (MeSH)
• Khan Academy, video lecture