What determines the amount of deflection in a mass spectrometer?
Mar 04, 2020 · What is deflection in mass spectrometry? The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. The amount of deflection also depends on the number of positive charges on the ion - in other …
What is the deflection of ions in a spectrophotometer?
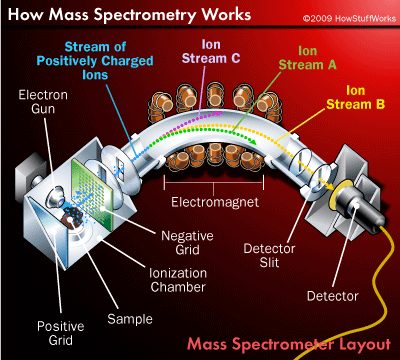
Mar 31, 2009 · Deflection and Detection of Ions. Creating and accelerating ions are essentially preparatory steps to the real work of mass spectrometry -- mass analysis. The main job of the mass analyzer is to apply an external magnetic field to the ions exiting the ionization chamber.
What determines the amount of deflection of ions by magnetic field?
Jan 05, 2022 · The speed at which they accelerate depends on their mass so the lighter ions move faster than the heavier molecules. Deflection In this stage, the stream of positively charged ions are deflected by a magnetic field. The extent of the deflection depends on the mass and …
What can be deflected by a magnetic field?
An outline of what happens in a mass spectrometer Atoms can be deflected by magnetic fields - provided the atom is first turned into an ion. Electrically charged particles are affected by a …
What causes deflection in mass spectrometry?
What does deflected mean in chemistry?
What are the 4 main stages in mass spectrometry?
- Ionization. The sample is vaporized before being passed into an ionization chamber where it is bombarded by a stream of electrons emitted by an electrically heated metal coil. ...
- Acceleration. ...
- Deflection. ...
- Detection.
What does mass spectrometry tell you?
What does deflection mean?
1 : a turning aside or off course : deviation. 2 : the departure of an indicator or pointer from the zero reading on the scale of an instrument.
What is deflection reason?
Why are lighter ions deflected more?
Why is a negatively charged plate used?
What is Cone voltage in mass spectrometry?
What is the difference between mass spectroscopy and mass spectrometry?
How does a mass spectrometer detect isotopes?
Does mass spectrometry destroy the sample?
How much does an ion's path curve depend on?
How much an ion's path curves depends on two factors: the mass of the ion and its charge. Lighter ions and ions with a greater charge are deflected more than heavier ions and ions with a smaller charge.
Which ion stream has the lightest particles?
The net result is that each ion follows a path dependent on its mass, as shown on the right. Ion stream A has the lightest particles and is deflected the most. Ion stream C has the heaviest particles and is deflected the least. The mass of the particles in ion stream B falls somewhere in between.
Where does the mass of particles in ion stream B fall?
The mass of the particles in ion stream B falls somewhere in between. Notice that only one of the ion streams actually passes through the mass analyzer and reaches the detection unit at the back of the device. The other two streams hit the side of the spectrometer and are neutralized.
How does mass analysis work?
The main job of the mass analyzer is to apply an external magnetic field to the ions exiting the ionization chamber. This external field interacts with the magnetic field generated by the fast-moving particles, causing the path of each particle to bend slightly. How much an ion's path curves depends on two factors: the mass of the ion and its charge. Lighter ions and ions with a greater charge are deflected more than heavier ions and ions with a smaller charge.
What is the main job of a mass analyzer?
The main job of the mass analyzer is to apply an external magnetic field to the ions exiting the ionization chamber. This external field interacts with the magnetic field generated by the fast-moving particles, causing the path of each particle to bend slightly.
What happens to a mass spectrometer?
An outline of what happens in a mass spectrometer. Atoms can be deflected by magnetic fields - provided the atom is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. Stage 1: Ionization: Gas phase particles of the sample are ionized through a collision ...
How are atoms deflected?
Atoms can be deflected by magnetic fields - provided the atom is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. Stage 1: Ionization: Gas phase particles of the sample are ionized through a collision with a high energy electron yielding a positive ion.
What stage of ionization is a gas phase particle?
Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. Stage 1: Ionization: Gas phase particles of the sample are ionized through a collision with a high energy electron yielding a positive ion.
What is the sequence of ionization?
The sequence is : Stage 1: Ionization: Gas phase particles of the sample are ionized through a collision with a high energy electron yielding a positive ion. Stage 2: Acceleration: The ions are accelerated so that they all have the same kinetic energy and directed into a mass analyzer.
What is the first stage of ionization?
Stage 1: Ionization: Gas phase particles of the sample are ionized through a collision with a high energy electron yielding a positive ion. Stage 2: Acceleration: The ions are accelerated so that they all have the same kinetic energy and directed into a mass analyzer.
What is the mean free path of an ionizer?
The mean free path is the average distance a particle travels before it suffers a collision with another particle.
What is the mean free path of a nitrogen molecule?
The mean free path is the average distance a particle travels before it suffers a collision with another particle. The mean free path is a concept often presented when discussing the Kinetic Molecular Theory in a first year chemistry course. The mean free path for a nitrogen molecule at room temperature and 1 atm pressure is 95 nm (9.5 x 10 -9 m). In a room temperature vacuum chamber the mean free path of a nitrogen molecule is 7.2 x 10 -5 m when the pressure is 1 mm Hg (1 Torr or 0.0013 atm), 7.2 x 10- 2 m when the pressure is 0.001 mm Hg (1 mTorr or 1.3 x 10 -6 atm), and 72 cm when the pressure is 1 x 10 -6 mm Hg (1 x 10 -6 Torr or 1.3 x 10 -9 atm). Given the typical dimensions of a mass analyzer and the larger cross section for collisions for an ion relative to a neutral molecule, mass spectrometers need to be operated under condition of high vaucuum, 10 -9 atm or lower.
How does a mass spectrometer work?
How a mass spectrometer works. If something is moving and you subject it to a sideways force, instead of moving in a straight line, it will move in a curve - deflected out of its original path by the sideways force. Suppose you had a cannonball traveling past you and you wanted to deflect it as it went by you.
How do atoms get deflected?
Atoms can be deflected by magnetic fields - provided the atom is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. Stage 1: Ionization: The atom is ionised by knocking one or more electrons off to give a positive ion.
What is the stage of mass spectrometer?
Mass spectrometers always work with positive ions. Stage 2: Acceleration: The ions are accelerated so that they all have the same kinetic energy. Stage 3: Deflection: The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected.
What is the stage 3 of ion deflection?
Stage 3: Deflection: The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. The amount of deflection also depends on the number of positive charges on the ion - in other words, on how many electrons were knocked off in the first stage.
What stage of a ion deflection depends on the number of positive charges?
The more the ion is charged, the more it gets deflected. Stage 4: Detection: The beam of ions passing through the machine is detected electrically.
How does stream B get removed from a mass spectrometer?
Eventually, they get removed from the mass spectrometer by the vacuum pump.
Which stage of spectrometers always work with positive ions?
This is true even for things which you would normally expect to form negative ions (chlorine, for example) or never form ions at all (argon, for example). Mass spectrometers always work with positive ions. Stage 2: Acceleration: The ions are accelerated so that they all have the same kinetic energy.
Who invented mass spectrometry?
John B. Fenn, the originator of electrospray ionization for biomolecules and the 2002 Nobel Laureate in Chemistry, probably gave the most apt answer to this question: Mass spectrometry is the art of measuring atoms and molecules to determine their molecular weight.
What are the components of a mass spectrometer?
Four basic components are, for the most part, standard in all mass spectrometers ( Figure 1.2 ): a sample inlet, an ionization source, a mass analyzer and an ion detector. Some instruments combine the sample inlet and the ionization source, while others combine the mass analyzer and the detector.
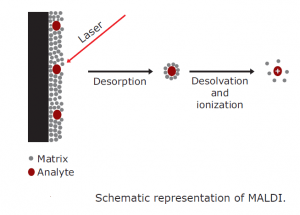
When was matrix assisted laser desorption/ionization mass spectrometry (MALDI-MS) first
Matrix-assisted laser desorption/ionization mass spectrometry (MALDI-MS) was first introduced in 1988 by Tanaka, Karas, and Hillenkamp. It has since become a widespread analytical tool for peptides, proteins, and most other biomolecules (oligonucleotides, carbohydrates, natural products, and lipids).
What is the ability of a mass spectrometer to distinguish between ions of different mass-to-charge
Resolution is the ability of a mass spectrometer to distinguish between ions of different mass-to-charge ratios. Therefore, greater resolution corresponds directly to the increased ability to differentiate ions. The most common definition of resolution is given by the following equation:
Why do mass spectrometers need vacuum?
All mass spectrometers need a vacuum to allow ions to reach the detector without colliding with other gaseous molecules or atoms. If such collisions did occur, the instrument would suffer from reduced resolution and sensitivity. Higher pressures may also cause high voltages to discharge to ground which can damage the instrument, its electronics, and/or the computer system running the mass spectrometer. An extreme leak, basically an implosion, can seriously damage a mass spectrometer by destroying electrostatic lenses, coating the optics with pump oil, and damaging the detector. In general, maintaining a good vacuum is crucial to obtaining high quality spectra.
What is a mass analyzer?
The mass analyzer is a critical component to the performance of any mass spectrometer. Among the most commonly used are the quadrupole, quadrupole ion trap, time-of-flight, time-of-flight reflectron, and FTMS. However, the list is growing as more specialized analyzers allow for more difficult questions to be addressed. For example, the development of the quad-TOF has demonstrated its superior capabilities in high accuracy tandem mass spectrometry experiments. Once the ions are separated by the mass analyzer they reach the ion detector, which is ultimately responsible for the signal we observe in the mass spectrum.
What is the smallest scale in the world?
Mass spectrometry has been described as the smallest scale in the world, not because of the mass spectrometer's size but because of the size of what it weighs -- molecules. Over the past decade, mass spectrometry has undergone tremendous technological improvements allowing for its application to proteins, peptides, carbohydrates, DNA, drugs, ...
What is mass spectrometry?
In a nutshell, mass spectrometry accurately measures the mass of different molecules within a sample. Even large biomolecules like proteins are identifiable by mass, which means that biologists can perform some very interesting experiments using mass spectrometry, potentially adding a new dimension to their research.
Why is mass spectrometry used to measure ions?
Because mass spectrometry measures the mass of charged particles, only ions will be detected, and neutral molecules will not be seen. Ions are created by giving electrons to a molecule (producing a negatively charged ion) or taking electrons away from a molecule (producing a positively charged ion). Note: A sample can only be analyzed by mass ...
What is mass spec?
Mass spectrometry, also referred to as mass spec or MS, is an analytical technique that is becoming increasingly important in bioscience research. This article will introduce you to mass spectrometry in biological research, explain how it works, and how it could be useful in your research.
Can biologists use mass spectrometry?
Even large biomolecules like proteins are identifiable by mass, which means that biologists can perform some very interesting experiments using mass spectrometry, potentially adding a new dimension to their research.
How are ions created in mass spectrometry?
Molecules in a sample are vaporized (converted to the gas phase by heating). Then, an electron beam bombards the vapors, which converts the vapors to ions. Because mass spectrometry measures the mass of charged particles, only ions will be detected, and neutral molecules will not be seen. Ions are created by giving electrons ...
What are the two stages of mass spectrometry?
Note: A sample can only be analyzed by mass spectrometry if it can be vaporized without decomposing. 2. Acceleration and Deflection. Next, the ions are sorted according to mass in two stages – acceleration and deflection. Acceleration is simply attraction.
Does mass spectrometry measure weight?
In a word, yes! Mass spectrometry provides accurate weight measurements for your bio- (or other) molecules, which can be used to: Give a good estimate of the purity of the sample (i.e. whether there are one or more molecular species in your sample and what ratio those species are in)
How does a mass spectrometer work?
The basic principle. If something is moving and you subject it to a sideways force, instead of moving in a straight line, it will move in a curve - deflected out of its original path by the sideways force.
What happens to a mass spectrometer?
An outline of what happens in a mass spectrometer. Atoms and molecules can be deflected by magnetic fields - provided the atom or molecule is first turned into an ion. Electrically charged particles are affected by a magnetic field although electrically neutral ones aren't. The sequence is : Stage 1: Ionisation.
How do ions get removed from the mass spectrometer?
Eventually, they get removed from the mass spectrometer by the vacuum pump. When an ion hits the metal box, its charge is neutralised by an electron jumping from the metal on to the ion (right hand diagram).
What is the first stage of ionization?
Stage 1: Ionisation. The atom or molecule is ionised by knocking one or more electrons off to give a positive ion. This is true even for things which you would normally expect to form negative ions (chlorine, for example) or never form ions at all (argon, for example). Most mass spectrometers work with positive ions.
How do atoms become ionized?
The atom or molecule is ionised by knocking one or more electrons off to give a positive ion. This is true even for things which you would normally expect to form negative ions (chlorine, for example) or never form ions at all (argon, for example). Most mass spectrometers work with positive ions.
What is the stage 3 of ion deflection?
Stage 3: Deflection. The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. The amount of deflection also depends on the number of positive charges on the ion - in other words, on how many electrons were knocked off in the first stage.
How are ions deflected?
The ions are then deflected by a magnetic field according to their masses. The lighter they are, the more they are deflected. The amount of deflection also depends on the number of positive charges on the ion - in other words, on how many electrons were knocked off in the first stage.