What are typical values for the dielectric constant?
Typical values of ε for dielectrics are: A dielectric constant of 2 means an insulator will absorb twice more electrical charge than vacuum. Use of materials in the production of capacitors used in radios and other electrical equipment. Commonly used by circuit designers to compare different printed-circuit-board (PCB) materials.
What are the units of the dielectric constant?
dielectric constant formula
- C = capacitance using the material as the dielectric capacitor
- C 0 = capacitance using vacuum as the dielectric
- ε 0 = Permittivity of free space (8.85 x 10 -12 F/m i.e. Farad per metre)
- A = Area of the plate/sample cross section area
- T = Thickness of the sample
What does dielectric constant mean?
The dielectric constant is the ratio of the permittivity of a substance to the permittivity of free space. It is an expression of the extent to which a material concentrates electric flux, and is the electrical equivalent of relative magnetic permeability. As the dielectric constant increases, the electric flux density increases, if all other factors remain unchanged.
What is the equation for dielectric constant?
The dielectric constant (k) of a material is the ratio of its permittivity ε to the permittivity of vacuum ε o , so k = ε / ε o. The dielectric constant is therefore also known as the relative permittivity of the material. Since the dielectric constant is just a ratio of two similar quantities, it is dimensionless.

Is dielectric constant of water is 20?
The dielectric constant k is the relative permittivity of a dielectric material. It is an important parameter in characterizing capacitors....Dielectric Constants at 20°C.MaterialDielectric ConstantWater80.4Glycerin42.5Liquid ammonia(-78°C25Benzene2.28414 more rows
What is dielectric constant for water and air?
The value of the dielectric constant at room temperature (25 °C, or 77 °F) is 1.00059 for air, 2.25 for paraffin, 78.2 for water, and about 2,000 for barium titanate (BaTiO3) when the electric field is applied perpendicularly to the principal axis of the crystal.
Is water have high dielectric constant?
Overview. Water is extremely unique in that it has a high dielectric constant (=80). A high dielectric constant suggests that the solvent (in this case, water) has the ability to screen charges.
How do you calculate dielectric constant of water?
Answer and Explanation: κ=ϵw/ϵ0 κ = ϵ w / ϵ 0 where ϵ0=1 ϵ 0 = 1 and ϵw=88 ϵ w = 88 at the temperature 0∘ or ϵw=80 ϵ w = 80 at the temperature 20∘ . As a result, we can conclude the dielectric constant of water, κw is 88 at the temperature 0∘ or it is 80 at the temperature 20∘ .
What is dielectric constant of oil?
Hydrocarbon lubricating oils have dielectric constants that typically range from 2.1 to 2.4, depending on the viscosity of the oil, the oil's density, the relative paraffinic, naphthenic, and aromatic content and the oil's additive package.
How is water a good dielectric?
Pure water is a very effective dielectric at high frequencies, though to keep it pure normally involves pumping it round an ion-exchange resin to remove the ions dissolving into it from the enclosure. It also has a very high breakdown voltage compared to air (50 million volts per meter or more).
Why is water dielectric constant so high?
Water molecules are always associated with each other through as many as four hydrogen bonds and this ordering of the structure of water greatly resists the random thermal motions. Indeed it is this hydrogen bonding which is responsible for its large dielectric constant.
What is meant by dielectric constant of water is 80?
Solution : By the statement we mean that the capacitance of a capacitor will become 80 times if water is used as dielectric instead of air between its plates.
Why the dielectric constant of water is as high as 81?
Water has a unsymmetrical space as compared to mica. Since it has a permanent dipole moment, it has a greater dielectric constant as compared to mica.
What is the unit of dielectric constant?
The dielectric constant of a substance is defined as the ratio of the substance's permittivity to the permittivity of free space. It can be written as K = ε ε ο . It is a unitless, dimensionless quantity because it is the ratio of two like entities.
Why is dielectric constant important?
The dielectric constant of a material, also called the permittivity of a material, represents the ability of a material to concentrate electrostatic lines of flux. In more practical terms, it represents the ability of a material to store electrical energy in the presence of an electric field.
Which has highest dielectric constant?
The highest dielectric constant is Calcium Copper Titanate. and goes deeper at higher frequencies. It used to develop supercapacitors. However, there are some limitations to its application, and it may take some time.
What is meant by dielectric constant of water is 80?
Solution : By the statement we mean that the capacitance of a capacitor will become 80 times if water is used as dielectric instead of air between its plates.
What is dielectric air strength?
The dielectric strength of air is 3.0 × 106 V / m.
Which has highest dielectric constant?
The highest dielectric constant is Calcium Copper Titanate. and goes deeper at higher frequencies. It used to develop supercapacitors. However, there are some limitations to its application, and it may take some time.
What does you mean by dielectric constant of water is 81?
Water's dielectric constant of 81 means that electric fields are reduced by 81 times in water compared with their values in vacuum. E= 1 / 81 times that of it's value in vacuum. If water is used as a dielectric between the Parallel plates Capacitor.
What is the dielectric constant of free space?
Dielectric constant is the ratio of given material permittivity to the permittivity of free space. Permittivity is the property of any material to pass the electric field lines through it. Permittivity is the proportionality constant of the applied electric field and the displacement occurs in the charge carriers on application of the electric field. In free space or vacuum its value is#N#8.85 × 1 0 − 12 F / m#N#8.85 times {10^ { - 12}}; {rm {F/m}} 8.85× 10−12 F/m (farad per meter). Mostly the electric permittivity is expressed in relation to the permittivity in vacuum as every material has different strength to pass the electric field lines through it.
What is a good dielectric material?
A good dielectric material is that which can polarize easily. Water is a very good dielectric material as it can polarize easily and have large value of dielectric constant. Dielectrics are used in capacitors, transmission lines etc. Permittivity can also be explained in terms of polarity as material having less permittivity can polarize ...
Why is permittivity important?
The permittivity represents how difficult it is for the field to porpagate inside a medium due to the response of the medium to the field. Imagine you are walking on a trampoline vs on asphalt , trampoline stretches while asphalt does not. It is much easier to walk on asphalt than trampoline. And the more stretchable the trampoline is the harder it is to walk on it. This is similar to the polarizability (stretching of opposite charges) . Higher polarizability results in higher permittivity (and dielectric constant) and the lower the speed of light .
What is the charge that is bound?
This charge is called the bound charge. The field from these bound charges is identical to the field from the dipoles - all we’ve really done is reframed how we think about it - but it’s mathematically easier to work with. The density of these bound charges depends on how quickly the dipole moments change through space.
How many atoms are in a water molecule?
On the atomic level, a molecule of water has a large Oxygen atom in the center, and two atoms of Hydrogen, one on either side of the Oxygen. However, because of the physics on the sub-atomic level, the stable location of the hydrogens are not on opposite sides of the Oxygen but are at an angle to the Oxygen, If you think of the Oxygen as being in the center of a Tetrahedron ( the simplest 4-sided solid),The Hydrogens occupy two points of the tetrahedron ( not really true, but makes visualization easier). Since the electrons from the Oxygen spend most of their time in the vicinity of the Hydrog
What is the dielectric constant of water?
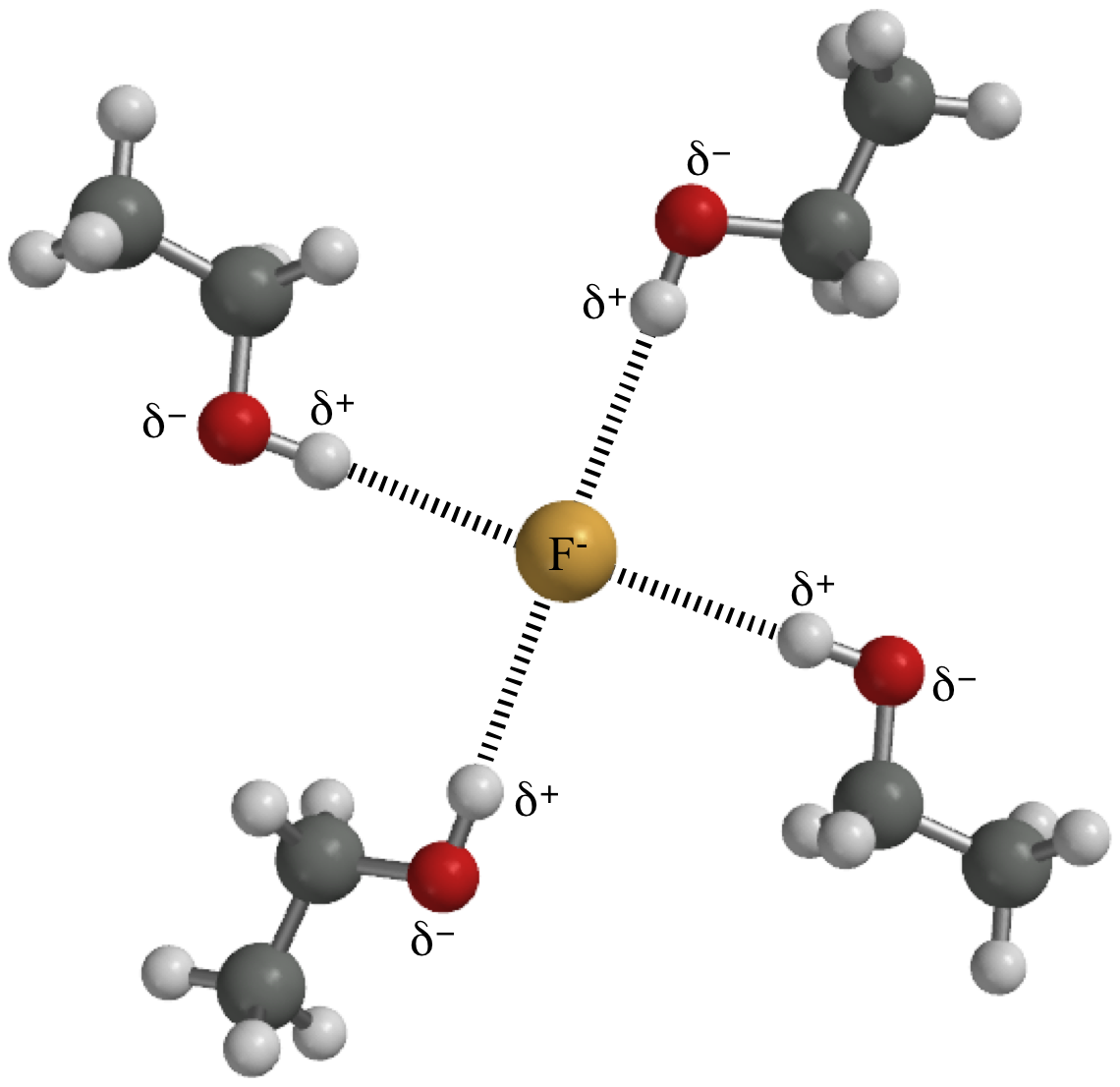
Water has a dielectric constant because it is made of dipoles as I mentioned earlier - in the case of water, this constant is around 80.
How does an electric field affect the speed of light?
this creates a separation in charge, that has its own field which interacts with the incident field. It slows it down, and it decreases the coulomb force between two charges in that material . The factor by which the force decreases is the dielectric constant. The factor by which the speed of light inside a dielectric slows down is the square root of the dielectric constant.
How to find the bound charge of a substance?
Luckily, there’s a bit of a trick. For many substances, the bound charge is proportional to the total charge, related by a dimensionless quantity χ, called the susceptibility. This makes sense - the total charge is what’s responsible for the electric field, and the electric field is what’s responsible for the bound charge, so we’d expect a relationship like this. This means we can write ρ b = − χ ( ρ b + ρ f), and therefore that ρ b = − χ 1 + χ ρ f. The negative sign is because the dipoles created by the electric field always oppose it.
What is a positive and negative charge a small distance away from each other called?
And what’s a positive and negative charge a small distance away from each other called? A dipole! And what do electric dipoles do? They create electric fields!
Some fluids and their dielectric constants or permittivities
The Dielectric Constant, or permittivity - ε - is a dimensionless constant that indicates how easy a material can be polarized by imposition of an electric field on an insulating material. The constant is
Engineering ToolBox - SketchUp Extension - Online 3D modeling!
Add standard and customized parametric components - like flange beams, lumbers, piping, stairs and more - to your Sketchup model with the Engineering ToolBox - SketchUp Extension - enabled for use with the amazing, fun and free SketchUp Make and SketchUp Pro .Add the Engineering ToolBox extension to your SketchUp from the SketchUp Pro Sketchup Extension Warehouse!.
Privacy
We don't collect information from our users. Only emails and answers are saved in our archive. Cookies are only used in the browser to improve user experience.
Advertise in the ToolBox
If you want to promote your products or services in the Engineering ToolBox - please use Google Adwords. You can target the Engineering ToolBox by using AdWords Managed Placements.
Citation
Engineering ToolBox, (2008). Dielectric Constants of Liquids. [online] Available at: https://www.engineeringtoolbox.com/liquid-dielectric-constants-d_1263.html [Accessed Day Mo. Year].
Why is the dielectric constant of water high?
The dielectric constant of water is still very high but we do not use it in the condenser. It is because of the presence of natural salts in the water. It has the presence of loosely bound and free-electron molecules present in water. 2.
What Is Dielectric Constant?
Many materials possess a tremendous property to hold their electrical charge for long intervals and in large quantities as well. Such property of materials refers to dielectric property. Many students get confused with the term dielectric constant, property, and often ask what is relative permittivity. This guide is beneficial to understand the meaning and factors affecting the dielectric constant.
What Are Dielectric Materials?
Dielectric materials have weak electrical conductivity but possess the ability to store an electrical charge. There are several dielectric materials, including vacuum, air, and more. The values of dielectric constants of some dielectric materials include:
What Do You Mean By Dielectric Constant Equation?
K is the ratio of two entities with the same dimension. Hence, the dielectric constant is a unitless and dimensionless quantity.
What Factors Affect The Value of The Dielectric Constant?
After knowing what is dielectric constant, there come different factors that affect the dielectric constant value such as:
What happens to the dielectric constant when the temperature goes above the transition temperature?
Moreover, if the temperature goes above the transition temperature, the dielectric constant will start decreasing.
Why is water considered a dielectric?
Ans: Water is a dielectric material due to polarisation. The higher polar molecules of water keep rotating, and thus it is an electric dipole. We generally consider it as dielectric because of high permittivity value at 20 degrees C. The dielectric constant of water is still very high but we do not use it in the condenser. It is because of the presence of natural salts in the water. It has the presence of loosely bound and free-electron molecules present in water.
What is the dielectric constant of a solvent?
A higher dielectric constant of the solvent correlates with a higher ability of the solvent to dissolve salts. This property is important when using buffers as eluant in HPLC. The dielectric constant of the solvent also affects interactions in solution that involve ions and polar molecules, decreasing the intermolecular energy when the dielectric constant increases, as shown by several formulas in Sections 4.1 and 4.2. The values of the dielectric constant for several common solvents at 25 o C are given in Table 7.2.1. The dielectric constant also depends on temperature. As an example, the variation of the dielectric constant of water as a function of temperature is shown in Figure 7.2.3.
How does the dielectric constant affect the intermolecular energy of a solution?
The dielectric constant of the solvent also affects interactions in solution that involve ions and polar molecules, decreasing the intermolecular energy when the dielectric constant increases , as shown by several formulas in Sections 4.1 and 4.2.
What happens to the permittivity of a polymer at higher temperatures?
At higher temperatures, the increased thermal vibrations cause the permittivity to drop again. If the frequency of the field is increased, the polar groups have less time to align; hence, the glass transition occurs at a higher temperature. This is shown in Fig. 1.25.
How does substrate affect relative permittivity?
electrodes), can affect the relative permittivity of materials, where the relative permittivity has both intrinsic and extrinsic contributions associated with the ferroelectric domains and domain wall motion. The constraining action of the substrate will prevent effective domain wall movement, thus significantly reducing the extrinsic contribution to relative permittivity. This constraint can lead to a degradation zone near to the interface, which can be described by a 2-2 structure.
What is the grain size of ferroelectric material?
Even in bulk ferroelectric ceramics, the grain size is known to affect the relative permittivity of the material with maximum properties reported for grain sizes in the 0.8–1-μm size range. This is a result of two phenomena that each contributes to relative permittivity:
How does the dielectric constant affect protein adsorption?
Dielectric constant of water is an important parameter affecting protein adsorption due to its influence on intermolecular forces. For instance, the strength of the electrical double layer is affected by the value of dielectric constant. The frequency response of dielectric constant proves important in determining the relative strength of van der Waals and electrostatic forces. Moreover, the role of water molecules in hydrophobic interactions and hydrogen bond formation are among other factors that need to be considered in protein adsorption, which is supported by a study showing that the structure of the water molecules constituting the surrounding hydration layer of a protein is a decisive factor with regard to the conformational states (native vs. unfolded).27
Does wood have a dielectric constant?
The dielectric constant of wood increases in direct proportion with density. As the frequency increases the dielectric constant diminishes, since fewer of the polar wood molecules have sufficient time to follow the external field changes (Torgovnikov 1993 ).
What is dielectric constant?
Dielectric constant, also called relative permittivity or specific inductive capacity, property of an electrical insulating material (a dielectric) equal to the ratio of the capacitance of a capacitor filled with the given material to the capacitance of an identical capacitor in a vacuum without the dielectric material.
What is the dielectric constant in a centimeter-gram-second system?
In the centimetre-gram-second system, the dielectric constant is identical to the permittivity. It denotes a large-scale property of dielectrics without specifying the electrical behaviour on the atomic scale.
Dielectric Constant Values
Dielectric Constant (k) is a number relating the ability of a material to carry alternating current to the ability of vacuum to carry alternating current. The capacitance created by the presence of the material is directly related to the Dielectric Constant of the material.
How to use this guide
CLIPPER CONTROLS has compiled an extensive list of products with Dielectric Constants. Many of these Dielectric Constants are given at specific temperatures. If your product's temperature is significantly different from those listed there is a good chance that the Dielectric Constant may be different from the values listed.
