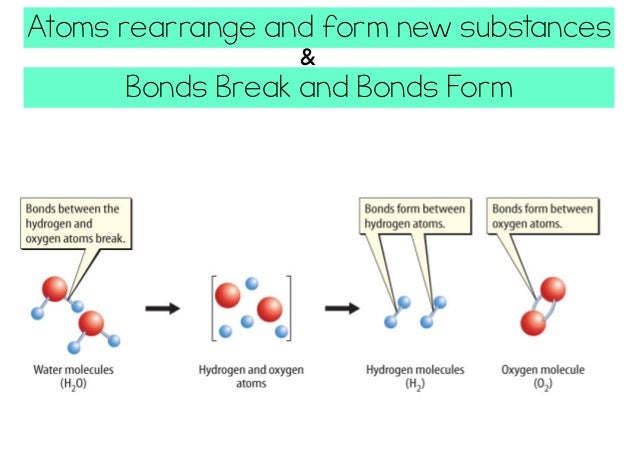
According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made.
When are bonds riskier than stocks?
Yes, stock prices fluctuate more than the prices of bonds but that doesn’t necessarily mean more risk for the investor. There are a lot of cases when bonds are riskier than stocks. For example, over a high inflationary period when inflation is surging quickly, the bond price can be damaged, decreased.
What energy is released when a chemical bond is broken?
cellular respiration. In cellular respiration, bonds are broken in glucose, and this releases the chemical energy that was stored in the glucose bonds. Some of this energy is converted to heat. The rest of the energy is used to form many small molecules of a compound called adenosine triphosphate, or ATP. See also when were paved roads invented.
Is energy released when bonds break?
Why is energy not released when bonds are broken? Energy is not released when the chemical bond between two atoms is broken. In fact, it takes the input of energy to break a bond. Does breaking a bond always release energy? Since breaking bonds requires adding energy, the opposite process of forming new bonds always releases energy. The stronger the bond formed, the more energy is released during the bond formation process.
Why is energy released when a bond is formed?
The lost energy is available to be “released” in another form. So that is the basic answer: creating bonds releases energy because the two atoms in a bond are attracted to each other. On the microscopic level, the release of energy usually takes place through a third atom or molecule.

What happens when bonds are broken?
When a chemical reaction occurs, molecular bonds are broken and other bonds are formed to make different molecules. For example, the bonds of two water molecules are broken to form hydrogen and oxygen. Energy is always required to break a bond, which is known as bond energy.
What type of reaction occurs to break a bond?
Chemical reactions that release energy are called exothermic. In exothermic reactions, more energy is released when the bonds are formed in the products than is used to break the bonds in the reactants. Exothermic reactions are accompanied by an increase in temperature of the reaction mixture.
What has to happen in order for bonds to break in reactants?
In reality, the reactants need to collide and interact with each other in order for their bonds to break and rearrange. Also, the animation shows all of the atoms in the reactants coming apart and rearranging to form the products.
What reaction occurs when the bonds of the reacting compounds are broken and new combinations are formed?
Chemical reactions occur when chemical bonds between atoms are formed or broken. The substances that go into a chemical reaction are called the reactants, and the substances produced at the end of the reaction are known as the products.
Is breaking bonds endothermic or exothermic?
endothermic processBond breaking is an endothermic process, because it requires energy. Bond forming is an exothermic process, because it releases energy. In reaction profile diagram, the energy change in a reaction, is the difference between the reactants and products.
Why does breaking bonds release energy?
The reason there is energy released in the process is because the products formed (ADP and hydrogenphosphate/phosphate) have stronger covalent bonds (plus intermolecular forces with the surrounding solution and dissolved ions) than the starting materials. This is the case for any exothermic process.
What happens when chemical bonds break and form?
A chemical reaction occurs when chemical bonds are broken and formed and atoms are exchanged to produce chemically different species. Both of these processes are chemical reactions.
Does breaking bonds release or absorb energy?
Since breaking bonds requires adding energy, the opposite process of forming new bonds always releases energy. The stronger the bond formed, the more energy is released during the bond formation process.
How do you tell if a bond is broken or formed?
0:2111:0117.07 Identifying Bonds Made and Broken - YouTubeYouTubeStart of suggested clipEnd of suggested clipMovements within a reaction is this idea of identifying bonds made in a reaction that is bondsMoreMovements within a reaction is this idea of identifying bonds made in a reaction that is bonds created in the products and bonds broken in a reaction that is bonds destroyed in the reactants.
What happens to chemical bonds during a chemical reaction?
According to the modern view of chemical reactions, bonds between atoms in the reactants must be broken, and the atoms or pieces of molecules are reassembled into products by forming new bonds. Energy is absorbed to break bonds, and energy is evolved as bonds are made.
Which statement describes the energy changes that occur as bonds are broken and formed?
Q. Which statement describes the energy changes that occur as bonds are broken and formed during a chemical reaction? Energy is absorbed when bonds are both broken and formed.
What is the force of attraction that breaks and reforms in a chemical reaction?
Bonds break in the reactants and new bonds form in the products. The reactants and products contain the same atoms, but they are rearranged during the reaction. As a result, the atoms are in different combinations in the products than they were in the reactants.
What is it called when a molecule breaks apart?
Dissociation is one of the simplest chemical reactions, where a molecule breaks apart into two or more fragments, i.e., other molecules, atoms, ions, or radicals.
Is this reaction exothermic or endothermic?
0:254:16What Are Endothermic & Exothermic Reactions | Chemistry | FuseSchoolYouTubeStart of suggested clipEnd of suggested clipAn exothermic reaction gives off energy to the surroundings. Like this fire giving off heat whereasMoreAn exothermic reaction gives off energy to the surroundings. Like this fire giving off heat whereas an endothermic reaction takes in energy from its surroundings.
Is bond breaking spontaneous?
Breaking Bonds Requires Energy After all, molecules don't spontaneously break. For example, when is the last time you saw a pile of wood spontaneously burst into flames or a bucket of water turn into hydrogen and oxygen? Energy must be applied for these reactions to occur.