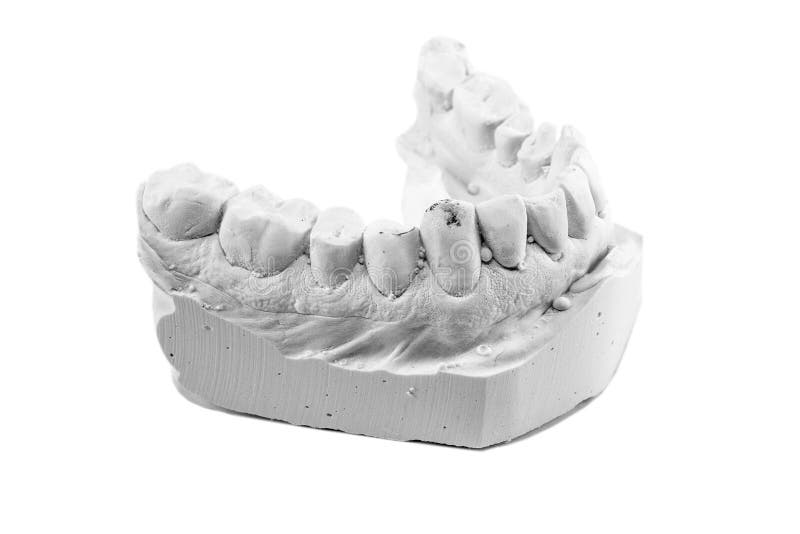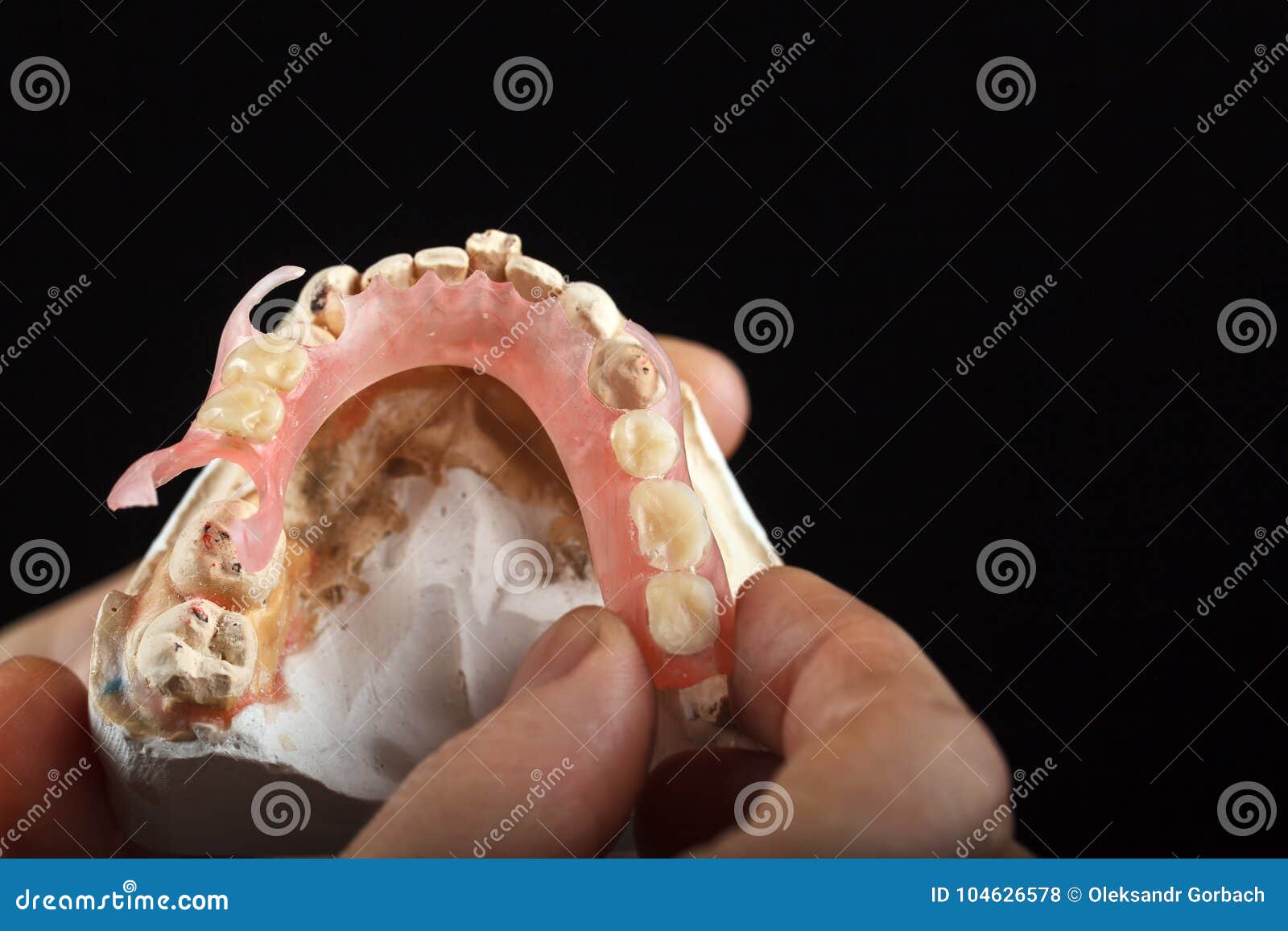
Gypsum is a white powdery mineral widely found in nature. Used for making dental casts since 1756. Chemical name Calcium sulphate dihydrate.(CaSO.2H2O) In dentistry, gypsum is used in the form of Calcium sulphate hemihydrate.
Why choose dental gypsum products?
With safety in mind, our dental gypsum products are designed to maintain their freshness for the long haul, making your purchase well worth the investment. The Hydrock Model Stone is ideal for partial or full denture models and other dental structures that require a reliable level of accuracy and durability.
What are the different types of gypsum products?
A2) Types of Gypsum products are: Type I – Impression Plaster. Type II – Dental Plaster. Type III – Dental Stone Type IV – Improved Dental Stone or Die stone or High Strength Stone. Type V – Dental Stone, High Strength, High Expansion. 18.
What is gypsum?
What is gypsum ? Name the varieties of gypsum. It is a natural mineral mined around the various parts of the world having a chemical formula, CaSO4·s2H2O (calcium sulphate dihydrate). TYPES:· Albaster:-pure white ,fine grained and translucent.
What do you mean by “hydrocal gypsum?
What do you mean by ‘hydrocal’?Give its uses. It is the type III gypsum product called DENTAL STONE used for model preparation. USES: For preparing master casts and to make moulds.

What is gypsum products in dentistry?
Gypsum products are one of the most broadly utilized dental materials for fabrication of dental casts and dies which are used as for further construction of indirect dental restorations. Gypsum products are obtained from natural gypsum minerals.
What is gypsum product?
Gypsum (hydrous calcium sulfate) is a popular raw material for manufacturing various construction products, such as plasters, drywall (wallboard or plasterboard), ceiling tiles, partitions, and building blocks.
What are the types of gypsum?
GypsumMajor varietiesSatin sparPearly, fibrous massesSeleniteTransparent and bladed crystalsAlabasterFine-grained, slightly colored30 more rows
Is dental plaster gypsum?
0:3513:48Gypsum Products | Dental Plaster Dental Stone | Dental MaterialsYouTubeStart of suggested clipEnd of suggested clipAnd then if you want to know what is gypsum basically gypsum is a mineral which is yellowish inMoreAnd then if you want to know what is gypsum basically gypsum is a mineral which is yellowish in color and it is present in many countries. And this gypsum is basically is in a dihydrate. Form so this
Why is dental plaster called gypsum?
Gypsum products used in dentistry are formed by driving off part of the water of crystallization from gypsum to form calcium sulphate hemihydrate. Plaster is produced by a process known as calcination.
What is gypsum used for?
Crude gypsum is used as a fluxing agent, fertilizer, filler in paper and textiles, and retarder in portland cement. About three-fourths of the total production is calcined for use as plaster of paris and as building materials in plaster, Keene's cement, board products, and tiles and blocks.
What are the three forms of gypsum?
the three varieties of gypsum are selenite, satin spar, and rock gypsum.
Is gypsum used in toothpaste?
Because of its binding abilities, gypsum is a primary ingredient in some toothpastes. It is also used as plaster to create surgical casts; as an additive in many foods, like canned vegetables, ice cream and tofu; and as an amendment, conditioner and fertilizer for agricultural applications.
What is the chemical name for gypsum?
calcium sulfate dihydrateGypsum is the name given to a mineral categorized as calcium sulfate mineral, and its chemical formula is calcium sulfate dihydrate, CaSO4⋅ 2H2O.
What material a dental plaster is?
gypsum mineralDental Plaster is a formulated hemihydrate plaster (CaSO4. 1/2 H2O) produced from naturally occurring gypsum mineral. It is off-white in colour. It is used in dentistry during the flasking operation in the production of dentures.
What is the difference between dental stone and dental plaster?
When choosing stone or plaster it is important to know the unique properties of both. Plaster is a less refined material and is distinguished microscopically by irregular shaped crystals. Plasters typically have higher water powder ratios of 40-50 milliliters per 100 grams of powder.
What is gypsum powder?
Gypsum is a naturally occurring soft sulfate mineral composed mainly of calcium sulfate dihydrate (chemical formula is CaSO4·2H2O). It is also known as drywall, sheetrock, wallboard or plasterboard. This powder is then made use of in various agricultural, industrial, and construction applications.
What is the chemical formula for gypsum?
Name the varieties of gypsum. It is a natural mineral mined around the various parts of the world having a chemical formula, CaSO4·s2H2O (calcium sulphate dihydrate). TYPES:· Albaster:-pure white ,fine grained and translucent.
Which substances increase the setting time of gypsum products by decreasing the rate of reaction?
E.g., Tera alba, sodium sulphate, potassium sulphate and sodium chloride (low concentration) RETARDERS:-They are the substances which increases the setting time of gypsum products by decreasing the rate of reaction. E.g., Borax, sodium chloride, sodium sulphate (high concentration) ,glue ,agar, coagulated blood. 17.
Where is gypsum found?
Commercial quantities of gypsum are found in the cities of Araripina and Grajaú in Brazil; in Pakistan, Jamaica, Iran (world's second largest producer), Thailand, Spain (the main producer in Europe), Germany, Italy, England, Ireland and Canada and the United States.
Where is gypsum deposited?
Gypsum is deposited from lake and sea water, as well as in hot springs, from volcanic vapors, and sulfate solutions in veins. Hydrothermal anhydrite in veins is commonly hydrated to gypsum by groundwater in near-surface exposures. It is often associated with the minerals halite and sulfur.
What is gypsum crystal?
It also forms some of the largest crystals found in nature, up to 12 m (39 ft) long, in the form of selenite.
Why is plaster of Paris called plaster of Paris?
Because the quarries of the Montmartre district of Paris have long furnished burnt gypsum ( calcined gypsum) used for various purposes, this dehydrated gypsum became known as plaster of Paris.
What is the chemical formula for gypsum?
Alabaster. Fine-grained, slightly colored. Gypsum is a soft sulfate mineral composed of calcium sulfate dihydrate, with the chemical formula CaSO. 4·2H2O. It is widely mined and is used as a fertilizer and as the main constituent in many forms of plaster, blackboard / sidewalk chalk, and drywall.
What is the legal limit for gypsum?
The Occupational Safety and Health Administration (OSHA) has set the legal limit ( permissible exposure limit) for gypsum exposure in the workplace as TWA 15 mg/m 3 for total exposure and TWA 5 mg/m 3 for respiratory exposure over an 8-hour workday.
What is the name of the white gypsum?
A massive fine-grained white or lightly tinted variety of gypsum, called alabaster, has been used for sculpture by many cultures including Ancient Egypt, Mesopotamia, Ancient Rome, the Byzantine Empire, and the Nottingham alabasters of Medieval England. Gypsum also crystallizes as translucent crystals of selenite.
What is gypsum used for?
Gypsum products probably serve the dental profession more adequately than any other materials. Dental plaster, stone, high-strength/high-expansion stone, and casting investment constitute this group of closely related products. With slight modification, gypsum products are used for several different purposes. For example, impression plaster is used to make impressions of edentulous mouths or to mount casts, whereas dental stone is used to form a die that duplicates the oral anatomy when poured into any type of impression. Gypsum products are also used as a binder for silica in gold alloy casting investment, soldering investment, and investment for low-melting-point nickel-chromium alloys. These products are also used as a mold material for processing complete dentures. The main reason for such diversified use is that the properties of gypsum materials can be easily modified by physical and chemical means.
Where is gypsum found?
Gypsum karst is widespread and well developed throughout the desert areas of southwestern USA (Nevada, Arizona, Utah, Wyoming, Colorado, New Mexico, and Texas), where it has formed on outcrops of Permian evaporites. Gypsum resists erosion in these areas and typically caps ridges, mesas, and buttes. Karst features are common, including caves, commonly with scalloped walls due to dissolution during high-flow events following flooding (Fig. 2B ). Dolines, karren, disappearing streams, springs, and collapse structures are also present ( Hill, 1996; Johnson, 2003, 2008 ). Of more than 500 known gypsum caves in the USA, most of them are in the southwestern part of the country ( Quinlan et al., 1986 ). Gypsum karst is most conspicuous in outcrops of gypsum, but also occurs where the gypsum is up to 30 m below the land surface.
What is the chemical formula for gypsum?
Gypsum is the name given to a mineral categorized as calcium sulfate mineral, and its chemical formula is calcium sulfate dihydrate, CaSO4 ⋅ 2H 2 O . However, a broader definition includes all the calcium sulfates, including calcium sulfate hemihydrate, CaSO 4 ⋅ 0.5H 2 O, which is known as plaster or plaster of Paris (POP). Figure 6 summarizes the polymorphism of calcium sulfate; ‘g’ indicates that the transformation reaction occurs in the gaseous phase, while ‘l’ indicates that the reaction occurs in the liquid phase. 23
How is gypsum board made?
Gypsum fiber boards are made by mixing gypsum with the reinforcing fiber in a relatively dry or slightly moist condition. Not much moisture can be added to the gypsum early in the process due to rapid setting of gypsum. In addition to gypsum and fibers, other materials are often incorporated into the mix (such as gypsum retarders or low-density aggregates) prior to board formation. Once the mixture has been formed into a mat, water is sprayed on this mat just prior to entering a press for mat consolidation. Press time is short due to rapid setting of the gypsum matrix. Once the board exits the press, additional curing and conditioning are performed ( Takahashi and Shigekura 1991 ). During the pressing, embossing can be done through the use of special dies.
Why is gypsum used in slip casting?
The gypsum mold is a critical element in slip casting, because the capillary suction of this porous material is responsible for slip dewatering (Dal and Berden 1968 ). Advantages of gypsum are the ease of its manufacture into a wide variety of shapes, low cost, good dimensional accuracy, and reproducible water absorption properties.
Can gypsum be converted to CaCO3?
The gypsum waste can be thermally reduced into CaS, which is then subjected to a direct aqueous carbonation step for the generation of H2 S and CaCO 3 [99]. CaS can be successfully converted into CaCO 3; however, the reaction may yield low-grade carbonate products (< 90% as CaCO 3) which comprise a mixture of calcite and vaterite, as well as trace minerals originating from the starting material. Thus, indirect aqueous CaS carbonation processing for the production of high-grade CaCO 3 (> 99% as CaCO 3) or precipitated CaCO 3 can be developed and optimized.
Where is gypsum found?
Since gypsum is found all over the world, its shape and texture also vary depending on which part of the world it is found. It is found in approximately 85 countries, and the biggest amount of gypsum is produced in North America.
What are some uses for gypsum?
Uses of Gypsums – Some of the Important Uses of Gypsums Include: Constructing buildings. In making pottery and moulds. In dental appliances to make casts and moulds and impression material. Manufacturing plaster of Paris. Conditioning soil. Hardening material in cement. Filler ingredient in many foods.
What are the components of gypsum?
The main components of gypsum are calcium sulfate (CaSO4) and water (H2O). Its chemical name is Calcium Sulphate Dihydrate and the chemical formula of gypsum is represented as CaSO4.2H2O. Gypsum and Anhydrite (CaSO4) are very similar chemically just that gypsum has 2 water molecules and Anhydrite does not contain any water molecules.
What is gypsum made of?
Gypsum is a naturally occurring mineral composed of hydrated calcium sulfate and appears soft white or grey in colour. It is formed mainly in layered sedimentary deposits and has a variety of uses in many industries like building, sculpting, gardening, and ornaments.
What is gypsum crystal?
Gypsum was known as Spear Stone in old English since it takes a crystal-like form, projecting out of a rock like a spear. You can mill mix gypsum with water to get its original rock-like shape, and it can be hardened. Its recycling loop can be termed as a “closed recycling loop” since you can recycle it for a number of times and it never loses its quality. It is moderately soluble in water, and its solubility reduces with the rise in temperature, contrary to the behaviour of other salts. Here are some of its important chemical and physical properties at a glance:
What are the different forms of gypsum?
Gypsum has found its use in diverse fields, based on which it can be classified in the below categories: Its Different Forms are: Rock in dull colour . Alabaster which is its fine-grained variety. Gypcrete or gypcrust, which is a hard layer formed on the soil.
What is the process of calcining gypsum?
It then goes through a process called calcining where heat at 350 degrees is supplied to the gypsum powder which removes 3/4th of the water molecules. Hemihydrate is the name of calcined gypsum which is then used in gypsum board, gypsum plaster, and other products.