What do you mean by changes in state of matter?
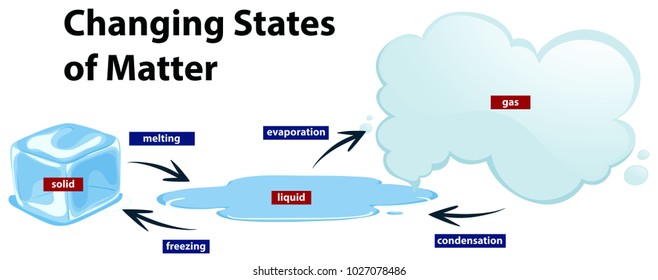
The processes by which a matter changes state or form are called : Melting, freezing, evaporation, condensation. The changes, from solid to liquid, from liquid to gas are reversible, and physical ( no chemical changes take place in the matter ) and are known as phases. Notes : Materials in general, when examined microscopically the phase or phases
What is the process by which a substance changes state called?
The process by which a substance changes from the solid phase to the liquid phase is known as melting. The process by which a substance changes from the liquid phase to the solid phase is known as freezing. The process by which a substance changes from the liquid phase to the gaseous phase is known as evaporation.
What is a change of State?
A change of state is a physical change in a matter. They are reversible changes and do not involve any changes in the chemical makeup of the matter. Common changes of the state include melting, freezing, sublimation, deposition, condensation, and vaporization.
How do you list the phase changes of matter?
Another way to list phase changes is by states of matter: Solids: Solids can melt into liquids or sublime into gases. Solids form by deposition from gases or freezing of liquids. Liquids: Liquids can vaporize into gases or freeze into solids.

When solids reach their melting point, what do they become?
Solids transform into liquid when they reach their melting point.
What is the boiling point?
Boiling point is defined as a temperature at which a pure liquid changes into a gas.
What is the melting point?
The melting point is defined as the temperature at which the solid starts to melt.
What is the process in which solids directly transform into a gas?
Sublimation is defined as the process in which the solid-state changes to a gaseous state without changing into a liquid state.
What is evaporation?
When the liquid gets converted to gas at all the temperatures, it is known as evaporation.
Why does matter change form?
You would have observed changing states of matter when ice cubes melt from solid into liquid water or when water boils into vapor, but have you wondered why substances change form? Changing states of matter occur when matter loses or absorbs energy. When a substance absorbs energy the atoms and molecules move more rapidly and this increased kinetic energy pushes particles far enough, that they change form. This energy is usually heat or thermal energy. In this article, let us understand the science behind the changing states of matter.
What are Changes of State?
Common changes of the state include melting, freezing, sublimation, deposition, condensation, and vaporization. These changes are shown in the figure given below.
What happens to the molecules when the temperature increases?
When the temperature or pressure increases, the interaction between the molecules increases. Similarly, when the temperature decreases, it is easier for molecules and atoms to settle into a more rigid structure. Freezing Melting Vaporization Condensation Sublimation Questions.
What is the process of a solid changing to a liquid called?
The process in which a solids change to a liquid is called melting. The melting point is the temperature at which a solids change to a liquid.
How does water change to gas?
This happens as particles of liquid water gain enough energy to completely overcome the force of attraction between them and change to the gaseous state. The bubbles rise through the water and escape from the pot as steam. The process in which a liquid boils and changes to a gas is called vaporization. The temperature at which a liquid boils is its boiling point.
What happens when you fill a pot with cold tap water and heat it on a hot stovetop?
If you fill a pot with cold tap water and heat it on a hot stovetop, the water heats up. Heat energy travels from the stovetop to the pot, and the water absorbs the energy from the pot. What happens to the water next?
What is the process of liquid water changing to solid ice called?
This way liquid water is changed into solid ice. The process of liquid water changing to solid ice is termed as freezing. The temperature at which it occurs is known as the freezing point.
How do states of matter change?
Another way to list phase changes is by states of matter: Solids: Solids can melt into liquids or sublime into gases. Solids form by deposition from gases or freezing of liquids. Liquids: Liquids can vaporize into gases or freeze into solids.
Why Do Phase Changes Occur?
Phase changes typically occur when the temperature or pressure of a system is altered. When temperature or pressure increases, molecules interact more with each other. When pressure increases or temperature decreases, it's easier for atoms and molecules to settle into a more rigid structure. When pressure is released, it's easier for particles to move away from each other.
How do liquids form?
Liquids form by condensation of gases and melting of solids. Gases: Gases can ionize into plasma, condense into liquids, or undergo deposition into solids. Gases form from the sublimation of solids, vaporization of liquids, and recombination of plasma. Plasma: Plasma can recombine to form a gas.
What is sublimation in chemistry?
Sublimation is the transition from a solid phase to a gas phase without passing through an intermediate liquid phase. Another example is when ice directly transitions into water vapor on a cold, windy winter day.
What is the white vapor that is observed in sublimation?
For example, if you view the sublimation of dry ice into carbon dioxide gas, the white vapor that is observed is mostly water that is condensing from water vapor in the air into fog droplets.
What is the opposite of evaporation?
This photo displays the process of condensation of water vapor into dew drops. Condensation, the opposite of evaporation, is the change in the state of matter from the gas phase to the liquid phase.
What is silver deposition?
Deposition is the settling of particles or sediment onto a surface. The particles may originate from a vapor, solution, suspension, or mixture. Deposition also refers to the phase change from gas to solid.