
How to solve for KC?
to calculate Kc K c you need the concentration of the reactants and products at equilibrium Table: A RICE table for mole values. Fill in the balanced chemical equation: aA+ bB ⇌ cC + dD aA + bB ⇌ cC + dD In the initial row fill in the number of moles of each substance present at the beginning of the reaction.
Why does constant KC in chemical equilibrium has no units?
The units of Equilibrium constant K will depend on the number of moles of reactants and products. Hence, it is concluded that equilibrium constant K has no units i.e. dimensionless if the total number of moles of products is equal to the total number of moles of reactants. So, the value of the equilibrium constant changes with temperature.
How to calculate equilibrium chemistry?
- The first step is to write down the balanced equation of the chemical reaction. aA +bB cC + dD
- The second step is to convert the concentration of the products and the reactants in terms of their Molarity.
- The third step is to form the ICE table and identify what quantities are given and what all needs to be found.
What is meant by KP and KC in chemistry?
What is meant by KP and KC in chemistry? K p And K c are the equilibrium constant of an ideal gaseous mixture. K p is equilibrium constant used when equilibrium concentrations are expressed in atmospheric pressure and K c is equilibrium constant used when equilibrium concentrations are expressed in molarity. 2A (g) +B (g) ⇋ 2C (g) All in the ...
What does KC stand for in chemistry equilibrium?
Kc = Equilibrium constant measured in moles per liter. Kp = Equilibrium constant calculated from the partial pressures.
What is KP and KC in chemical equilibrium?
Kp And Kc are the equilibrium constant of an ideal gaseous mixture. Kp is equilibrium constant used when equilibrium concentrations are expressed in atmospheric pressure and Kc is equilibrium constant used when equilibrium concentrations are expressed in molarity. For many general chemical reactions aA + bB ⇋ cC + dD.
How do u calculate KC?
Formula for Kc: The formula for Kc is Kc=[C]c[D]d[A]a[B]b K c = [ C ] c [ D ] d [ A ] a [ B ] b , where [C] and [D] are the molar concentrations of the products at equilibrium, and [A] and [B] are the molar concentrations of the reactants at equilibrium.
What does the KC value represent?
1 : The equilibrium constant Kc is a constant which represents how far the reaction will proceed at a given temperature. 8.2. 2 : When Kc is greater than 1, products exceed reactants (at equilibrium). When much greater than 1, the reaction goes almost to completion.
What is equilibrium constant K?
Equilibrium constant (K) - A mathematical ratio that shows the concentrations of the products divided by the concentrations of the reactants.
Are KC and Kp equal?
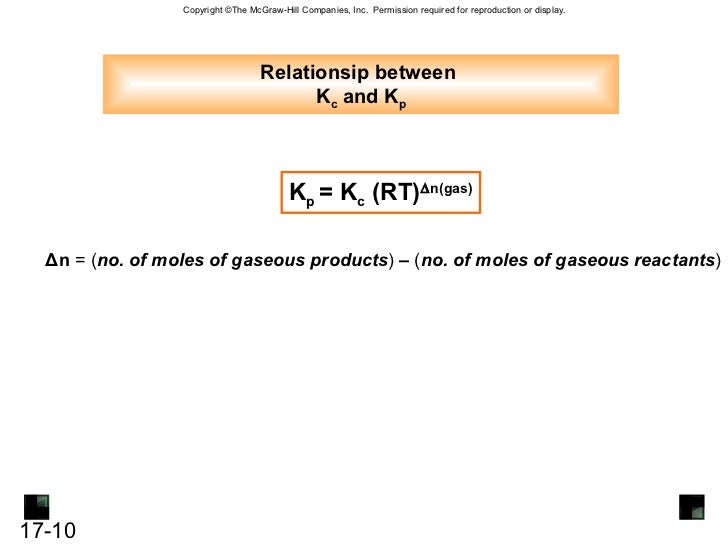
The general expression: Kp = Kc(RT) ∆n can be derived where ∆n = moles of gaseous products - moles of gaseous reactants.
What affects KC value?
Changing the temperature is the only factor that changes the value of Kc for a given equilibrium. When the concentration of a product is increased, the reaction proceeds in reverse to decrease the concentration of the products.
Does KC have a unit?
The units for Kc changes depending on the concentrations of each reactant and product in the equation. It can also be possible that there are no units for Kc because the concentrations at the top and the bottom both cancel eachother out.
How do you calculate the equilibrium constant KC?
0:528:12Worked examples: Calculating equilibrium constants | AP ChemistryYouTubeStart of suggested clipEnd of suggested clipWe start by writing the equilibrium constant which is symbolized by k and since we're dealing withMoreWe start by writing the equilibrium constant which is symbolized by k and since we're dealing with concentrations. We're calculating kc. And kc is equal to we do products over reactants.
What does K constant mean?
The rate constant, k, is a proportionality constant that indicates the relationship between the molar concentration of reactants and the rate of a chemical reaction. The rate constant may be found experimentally, using the molar concentrations of the reactants and the order of reaction.
Why is the equilibrium constant important?
The equilibrium constant is important because it gives us an idea of where the equilibrium lies. The larger the equilibrium constant, the further the equilibrium lies toward the products.
Why does KC depend on temperature?
This is because if you increase the temp., you drive the equilibrium backwards (in the endothermic direction), and therefore increase the concentration of reactants and decrease the concentration of products. If endothermic, increasing the temperature will increase Kc, and vice-verca.
How are KC and Kp related to each other in the reaction?
Hence, Kp=Kc.
What is Kp value in chemistry?
Summary. Equilibrium constant Kp is equal to the partial pressure of products divided by partial pressure of reactants and the partial pressure are raised with some power which is equal to the coefficient of the substance in balanced equation.
What is the relationship between Kp and KC for the reaction below?
Answer. Kp=Kc(RT)ⁿ where R is the gas constant, T is the Temperature and n is the change in no. of gaseous moles in the reaction.
What is the unit of KC?
The equation for Kc is [PRODUCTS]/[REACTANTS]. Hypothetically, if the equation was: A+ B --> C + 2D, the Kc equation would become: [C] [D]2 / [A] [B]. You would then replace the letters with the unit for concentration which is moldm-3 so it becomes: [moldm-3] [moldm-3 ]2/ [moldm-3] [moldm-3].
What is Equilibrium Constant?
The equilibrium constant of a chemical reaction (usually denoted by the symbol K) provides insight into the relationship between the products and reactants when a chemical reaction reaches equilibrium. For example, the equilibrium constant of concentration (denoted by K c) of a chemical reaction at equilibrium can be defined as the ratio of the concentration of products to the concentration of the reactants, each raised to their respective stoichiometric coefficients. It is important to note that there are several different types of equilibrium constants that provide relationships between the products and the reactants of equilibrium reactions in terms of different units.
What happens to the equilibrium constant of a reaction?
Simultaneous equilibrium reactions, having a common product. The equilibrium constant of the reactions does not change. Due to the higher concentration of the common product, the product concentrations will be reduced.
What is the equilibrium constant of stepwise multiple equilibria leading to the final products?
In case stepwise multiple equilibria leading to the final products, the equilibrium constant of the net equilibrium = product of each stepwise equilibrium constants. Therefore, the net equilibrium constant K = K 1 × K 2 × K 3.
How to calculate degree of dissociation of an equilibrium involving gas?
Degree of dissociation of an equilibrium involving gas can be calculated by knowing the equilibrium constant and the concentration of the gaseous reactant/product.
What happens to the equilibrium constant when the stoichiometry is changed?
If the equilibrium reaction stoichiometry is changed, the power of the equilibrium constant also gets changed by the same quantity.
What is the R value of C in mol dm 3?
If C is in mol dm 3 and p is in bar, then R = 0.0831 bar dm 3 mol -1 K -1 or p = CRT
What is the rate of the forward reaction?
At equilibrium, Rate of the forward reaction = Rate of the backward reaction
What is the equilibrium constant of a chemical reaction?
The equilibrium constant Kc in chemistry or Keq indicates the ratio of the concentration of all substances involved in a chemical equilibrium reaction . It is used in particular in connection with the law of mass action. That is why it is often referred to as the mass action constant, more rarely as the equilibrium constant.
Is mass action constant the equilibrium constant?
That is why it is often referred to as the mass action constant, more rarely as the equilibrium constant. In contrast to the position of the equilibrium, the value of the equilibrium constant depends only on the temperature and not on the concentration or the pressure.
What does it mean when all the powers in the equilibrium constant expression are 1?
As long as you keep the temperature the same, whatever proportions of acid and alcohol you mix together, once equilibrium is reached, Kcalways has the same value.
What is the equilibrium produced by heating carbon with steam?
The equilibrium produced on heating carbon with steam. Everything is exactly the same as before in the equilibrium constant expression, except that you leave out the solid carbon. The equilibrium produced between copper and silver ions. Both the copper on the left-hand side and the silver on the right are solids.
What are some examples of heterogeneous equilibrium?
A heterogeneous equilibriumhas things present in more than one phase. The usual examples include reactions involving solids and gases, or solids and liquids. Kcin homogeneous equilibria.
Why do we need to look at two different types of equilibria?
We need to look at two different types of equilibria (homogeneous and heterogeneous) separately, because the equilibrium constants are defined differently. A homogeneous equilibriumhas everything present in the same phase. The usual examples include reactions where everything is a gas, or everything is present in the same solution.
Is carbon dioxide a solid or a solid?
The only thing in this equilibrium which isn't a solid is the carbon dioxide.
How to find the Kc of an equilibrium reaction?
In an equilibrium reaction, Kc is obtained by multiplying the concentrations of products together, dividing the concentrations of the reactants and raising each concentration term to a power equal to the coefficient in the chemical equation. From the magnitude of Kc, you can tell whether a particular equilibrium favors products or reactants. The Qc refers to the reaction quotient and it’s value is compared with Kc. The reaction quotient,Qc has the same form as the equilibrium constant, Kc but it’s concentration values are not necessarily those at equilibrium.
What is the Qc in chemical equilibrium?
In chemical equilibrium Qc is called reaction quotient. It is just like the virtual equilibrium constant. It is a quantity which is considered before or after the equilibrium state .
What is the difference between chemical and physical equilibrium?
The difference between physical and chemical equilibrium is that a physical equilibrium is an equilibrium in which the physical state of the system does not change whereas chemical equilibrium is the equilibrium state in which the concentrations of reactants and products is not changed with time.
What is the equilibrium state in which the concentrations of reactants and products are not changed with time?
Chemical equilibrium is the equilibrium state in which the concentrations of reactants and products is not changed with time.
When Q is greater than KSP, what happens?
When Q (ion product constant) is greater than Ksp (solubility product constant) , you have a supersaturated solution and precipitation occurs.
Does physical equilibrium show change in physical states of matter?
Physical equilibriums show no change in physical states of matter that is involved in the equilibrium.
Does a change in reaction rate cause a shift in equilibrium?
Yes, a change in reaction rate is exactly why the shift in equilibrium happens.

What Is Equilibrium constant?
Equilibrium Constant Formula
- Kequ = kf/kb = [C]c [D]d/[A]a [B]b = Kc where Kc, indicates the equilibrium constant measured in moles per litre. For reactions involving gases:The equilibrium constant formula, in terms of partial pressure will be: Kequ = kf/kb = [[pC]c [pD]d]/[[pA]a [pB]b] = Kp Where Kp indicates the equilibrium constant formula in terms of partial pressures. 1. Larger Kc/Kp values indicate highe…
Units of Equilibrium Constant
- Equilibrium constant being the ratio of the concentrations raise to the stoichiometric coefficients. Therefore, the unit of the equilibrium constant = [Mole L-1]△n. where, ∆n = sum of stoichiometric coefficients of products – sum of stoichiometric coefficients of reactants. ⇒ Also Read: 1. Chemical Equilibrium 2. Ionic Equilibrium 3. Le Chatelier’s Principle
Equilibrium Constant, Reaction Quotient and Gibbs Free Energy
- K is the ratio of the relative amount of products to reactants at equilibrium while Q is the ratio at any point of time of the reaction. The Q value can be compared to K to determine the direction of the reaction to take place. The spontaneity of the process is related to the free energy change. △G (Gibbs Free Energy), K (Equilibrium Constant), and Q (Reaction Quotient) are related as foll…
Relationship Between KC and Kp
- Consider the following reversible reaction: cC + dD ⇒ aA + bB The equilibrium constant for the reaction expressed in terms of the concentration (mole/litre): If the equilibrium involves gaseous species, then the concentrations are replaced by partial pressures of the gaseous substances. The equilibrium constant in terms of partial pressures is: WherepA, pB, pC and pDrepresents th…
Characteristics of Equilibrium Constant
- It is reaction specific and at a constant temperature, it is fixed.
- A catalyst changes the rate of forward and backward reactions equally not to affect the value of the equilibrium constant.
- Changes in concentration, pressure, temperature, inert gasesmay affect the equilibrium, favoring either forward or backward reaction but not the equilibrium constant.
- It is reaction specific and at a constant temperature, it is fixed.
- A catalyst changes the rate of forward and backward reactions equally not to affect the value of the equilibrium constant.
- Changes in concentration, pressure, temperature, inert gasesmay affect the equilibrium, favoring either forward or backward reaction but not the equilibrium constant.
- Is related to the standard free energy as, △G0 = -RT ln Kequ.
Applications of Equilibrium Constant
- Equilibrium Constant For Predicting the Extent of Reaction
The equilibrium constant (Kc) can be used to predict the extent of a reaction, i.e. the degree of the disappearance of the reactants. The magnitude of the equilibrium constant gives an idea of the relative amount of the reactants and the products. Case 1: The larger value of the equilibrium co… - Equilibrium Constant for Predicting the Direction of a Reaction
The equilibrium constant can be used to predict the direction of the reaction. We need a term, reaction quotient (Qc expressed in terms of concentrations or Qp in terms of partial pressures) similar to the equilibrium constant except that the conditions are not at equilibrium. For a balanc…
Calculating The Equilibrium Concentration
- 1. From Equilibrium Constant:
Degree of dissociation of an equilibrium involving gas can be calculated by knowing the equilibrium constant and the concentration of the gaseous reactant/product. For example, in the decomposition of carbonate, the number of moles of carbon dioxide can be calculated from the e…
Factors Affecting Equilibrium Constant
- Some factors that affect equilibrium constant are: 1. Change in concentration of any product or reactant. 2. Change in the pressure of the system. 3. Change in temperature of the system. 4. Adding inert gas. 5. Adding catalyst.