KM is defined as the that results in half-maximal reaction rate. Vmax and KM are the two parameters which define the kinetic behavior of an enzyme as a function of. Vmax is a rate of reaction.
Is there a relationship between Vmax and km?
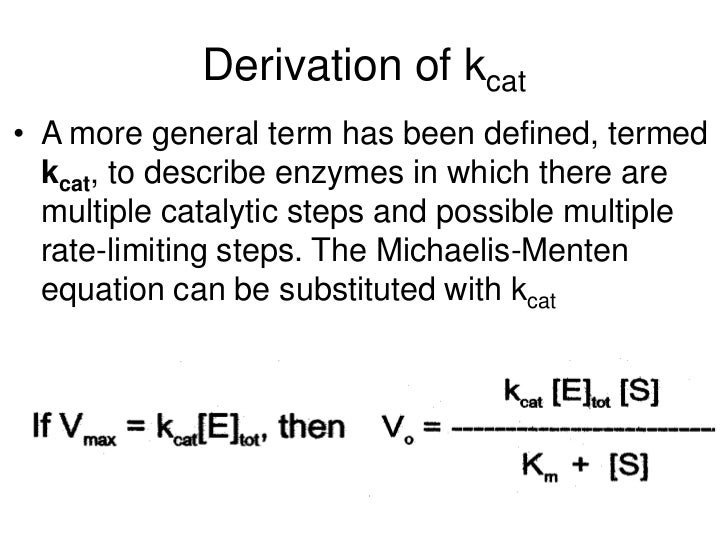
Vmax is equal to the product of the catalyst rate constant (kcat) and the concentration of the enzyme. Km is the concentration of substrates when the reaction reaches half of Vmax. A small Km indicates high affinity since it means the reaction can reach half of Vmax in a small number of substrate concentration.
What is the difference between kcat and Vmax?
What is the difference between Vmax and kcat? Vmax & Kcat. To determine Kcat, one must obviously know the Vmax at a particular concentration of enzyme, but the beauty of the term is that it is a measure of velocity independent of enzyme concentration, thanks to the term in the denominator. Kcat is thus a constant for an enzyme under given ...
What is the difference between kcat and km?
kcat is the turnover number, the number of times each enzyme site converts substrate to product per unit time. Km is the Michaelis-Menten constant, in the same units as X. It is the substrate concentration needed to achieve a half-maximum enzyme velocity. Et is the concentration of enzyme catalytic sites. Similarly, what is a good kcat value?
How to get Vmax?
Measuring KM and Vmax
- Run a series of reactions with constant [Etot], varying [S], and measure Vo.
- Graph Vo vs. [S].
- Estimate Vmax from asymptote.
- Calculate Vmax/2
- read KM from graph.

What is the definition of Vmax?
Vmax is the reaction rate when the enzyme is fully saturated by substrate, indicating that all the binding sites are being constantly reoccupied. From: Introduction to Biological and Small Molecule Drug Research and Development, 2013.
What is Km and Vmax in Michaelis Menten equation?
The Michaelis-Menten equation for this system is: Here, Vmax represents the maximum velocity achieved by the system, at maximum (saturating) substrate concentrations. KM (the Michaelis constant; sometimes represented as KS instead) is the substrate concentration at which the reaction velocity is 50% of the Vmax.
What is km of a reaction?
The higher the Kcat is, the more substrates get turned over in one second. Km is the concentration of substrates when the reaction reaches half of Vmax. A small Km indicates high affinity since it means the reaction can reach half of Vmax in a small number of substrate concentration.
What is Km value in Michaelis-Menten?
Km is a value obtained from v/[S] plots, and is equivalent to the concentration that provides 50% of Vmax ([S]0.5). The classical mechanistic model (eq. 3) assumes that i) steady state occurs for each [S] and ii) [enzyme] is negligible (<1000·[S]).
What is the unit for Vmax?
Vmax is a rate, and has units of inverse time built into it; its units are molarity/time, e.g. mol/L-s or mol/L-min. [E] is a concentration, i.e. units of mol/L.
What unit is Km?
kilometre (km), also spelled kilometer, unit of length equal to 1,000 metres and the equivalent of 0.6214 mile (see metric system).
What is Michaelis Menten used for?
The Michaelis–Menten equation is mainly used to characterize the enzymatic rate at different substrate concentrations, but it is also widely applied to characterize the elimination of chemical (the first-order kinetics) compounds from the body.
Does Km and Vmax have units?
Vmax is the maximum enzyme velocity in the same units as Y. It is the velocity of the enzyme extrapolated to very high concentrations of substrate, so its value is almost always higher than any velocity measured in your experiment. Km is the Michaelis-Menten constant, in the same units as X.
How do you read Vmax and Km?
The terms Km and Vmax are important in enzymatic kinetics. The key difference between Km and Vmax is that Km measures how easily the enzyme can be saturated by the substrate, whereas Vmax is the maximum rate at which an enzyme is catalyzed when the enzyme is saturated by the substrate.
What does high Vmax mean?
Biomolecules: Enzymes This point is reached when there are enough substrate molecules to completely fill (saturate) the enzyme's active sites. The maximal velocity, or Vmax, is the rate of the reaction under these conditions. Vmax reflects how fast the enzyme can catalyze the reaction.
Does Km and Vmax have units?
Vmax is the maximum enzyme velocity in the same units as Y. It is the velocity of the enzyme extrapolated to very high concentrations of substrate, so its value is almost always higher than any velocity measured in your experiment. Km is the Michaelis-Menten constant, in the same units as X.
What happens to Vmax and Km in noncompetitive inhibition?
The decrease in Vmax and the unchanged Km is the primary way to differentiate noncompetitive inhibition from competitive (no direct change in Vmax, increased Km) and uncompetitive (decreased Vmax and Km).
What does Hanes Woolf plot show?
The Hanes-Woolf plot is another method that linearises the Michaelis-Menten equation, plotting [S] (x-axis) against [S]/ν (y-axis). Just like the Lineweaver-Burk equation, the Hanes-Woolf equation is of the form y = mx + c.
How do you calculate Km of data?
From the graph find the maximum velocity and half it i.e. Vmax/2. Draw a horizontal line from this point till you find the point on the graph that corresponds to it and read off the substrate concentration at that point. This will give the value of Km.
What is a Hanes plot in biochemistry?
In biochemistry, a Hanes–Woolf plot, or better a Hanes plot, or better still a plot of a / v against a, is a graphical representation of enzyme kinetics in which the ratio of the initial substrate concentration a to the reaction velocity v is plotted against a. It is based on the rearrangement of the Michaelis–Menten equation shown below:
Why is the maximum velocity of enzymes called the maximum velocity?
This is referred to as the maximal velocity because the enzymes are working as fast as possible and will not respond to the addition of more substrate.
What does a large mean in enzyme kinetics?
This also indicates that the enzyme and substrate have high affinity for one another. A large indicates that a large amount of substrate is needed for the enzyme to become saturated and thus for the reaction to reach maximum velocity.
Which glucose transporter has the lowest affinity for glucose?
These glucose transporters rapidly take up glucose from the blood but have the lowest value. GLUT2 is commonly found in the liver and the pancreas. GLUT2 has a lower affinity for glucose but has the highest value. GLUT4 is common in skeletal tissues and in adipose tissues.
Is uncompetitive inhibition allosteric?
Uncompetitive inhibition occurs when an inhibitor binds to an allosteric site of a enzyme, but only when the substrate is already bound to the active site. In other words, an uncompetitive inhibitor can only bind to the enzyme-substrate complex.
Is allosteric inhibition reversible?
The inhibition can be reversed when the inhibitor is removed. … This is sometimes called allosteric inhibition (allosteric means ‘another place’ because the inhibitor binds to a different place on the enzyme than the active site).
How do you calculate Vmax?
The rate of reaction when the enzyme is saturated with substrate is the maximum rate of reaction, Vmax. … plotting v against v / gives a straight line:
How do you calculate Km and Vmax on a graph?
From the graph find the maximum velocity and half it i.e. Vmax/2. Draw a horizontal line from this point till you find the point on the graph that corresponds to it and read off the substrate concentration at that point. This will give the value of Km.
What is Km value?
Km (Michaelis Menten) indicates that substrate concentration attains half its maximum velocity when enzymes catalyze the chemical reaction. Km values generally lies between 10-1 to 10 -6M.
Do noncompetitive inhibitors affect Vmax?
For the competitive inhibitor, Vmax is the same as for the normal enzyme, but Km is larger. For the noncompetitive inhibitor, Vmax is lower than for the normal enzyme, but Km is the same.
Is noncompetitive inhibition reversible?
In noncompetitive inhibition, which also is reversible, the inhibitor and substrate can bind simultaneously to an enzyme molecule at different binding sites (see Figure 8.16). … Noncompetitive inhibition, in contrast with competitive inhibition, cannot be overcome by increasing the substrate concentration.
How does aerodynamic drag affect Vmax?from sciencedirect.com
Aerodynamic drag has a major effect on steady state Vmax performance as it is the major force to overcome at very high speed and it is generally perceived to be similarly important for aggressive track driving. The results show that for a 10% increase in drag coefficient the thermal effect around the Nurburgring is negligible with an increase of only 0.2 °C in fluid temperatures and 0.5s on lap time. There are two reasons for this. Firstly the average speed around the Nurburgring for the vehicles considered is about 85 mph and there are few places where speed exceeds 120 mph. Even on the long straight where drag becomes really significant speed is usually limited (not by drag) to 155 mph. Secondly the vehicles being considered have up to 500 hp available so the drag force at the average speed requires only a small proportion of the power available (about 10-15%), most of which is used to overcome vehicle inertia forces during acceleration. For lower powered vehicles which would spend a significant proportion of the lap at a speed limited by drag the effect would be much greater.
How to determine enzyme kinetic constant?from sciencedirect.com
Enzyme kinetic constants (K m and V max) are determined using initial velocity measurements obtained at varying substrate concentrations. All conditions (pH, temperature, enzyme concentration) are kept constant. Only the substrate concentration is allowed to vary. To obtain a wide range of velocities, the substrates are usually varied from 0.25 to 5 K m values. The widespread availability of personal computer curve-fitting programs has been of great benefit in determining enzyme kinetic constants. Such programs include SigmaPlot and KaleidaGraph. It is also possible to use the Microsoft Excel program to perform nonlinear least-squares fitting, provided that additional steps (macros) are used to generate the standard deviations and errors Billo (2001).
Can an advantage be taken with the generalities of the activation mechanism?from sciencedirect.com
Advantage can be taken with the generalities of the activation mechanism to introduce a component X to the activation mixture without specifying the properties of component X. Adjusting the subscripts accordingly, the relevant equations for component X are
Is Vmax proportional to protein abundance?from sciencedirect.com
Assuming Vmax is directly proportional to protein abundance and Km remains constant between in vitro models and in vivo (Fig. 1 ), the ratio of protein expression in tissues and in vitro models can be utilized as a scaling factor for IVIVE. Reliability of UGT proteomic quantification in liver microsomes was demonstrated by high correlation between protein expression and activity [79]. Similarly, significant correlation between UGT protein abundance and activity in human kidney microsomes has been shown, which was used to predict renal glucuronidation [112]. Successful IVIVE was reported for OCT2 transporter in predicting metformin renal clearance by incorporating plasma membrane abundance [113]. Mechanistic in vitro transporter data can be scaled up to predict in vivo disposition, as illustrated with testosterone glucuronide and efflux transporters MRP2 and MRP3 [114]. Although proteomics-informed IVIVE is promising, it is important to address sample preparation differences and the nature of the in vitro model. For example, for IVIVE efflux transport from vesicles, the %inside out should be measured experimentally to account for inactive transporters [114]. In overexpressing cells, a significant fraction of transporter could be present intracellularly (i.e., in trafficking process), which should be characterized to avoid overprediction of drug clearance [8, 9, 113]. These examples illustrate that proteomics-informed absolute scaling factor (ASF) approach can be used for accurate IVIVE, when consistency in sample extraction, recovery, and digestion are experimentally confirmed [14].
How are Km and Vmax related?
Km and Vmax are related to enzyme kinetics in a biological system. Km is the substrate concentration that is required for the reaction to occur at 1/2 Vmax. In other words, it is how much substrate is needed for the reaction to occur at 1/2 its max possible rate. Obviously Vmax is the maximum rate that the reaction can proceed at.#N#The reason Km increases with a competitive inhibitor is because the inhibitor is directly competing with the substrate for a fixed number of active sites on enzymes. Hence, Km increases. If there is a competitive inhibitor you will need more substrate to get the same 1/2 Vmax (this is why Km increases).#N#Vmax decreases with a noncompetitive inhibitor because the inhibitor does not bind to the active site. It binds allosterically so it is not competing directly. Its almost as if the substrate cannot win this binding race. So overall the reaction will not occur as fast as it could.
Why is the Vmax higher?
This is concentration dependent. The more enzymes you have, the higher the Vmax because even more enzyme's are doing their thing. Now, knowing this information we can apply it to certain inhibitors. Noncompetitive inhibitors bind else where on the enzyme.
What does it mean when a Km is small?
Don't memorize here... realize if Km is small, that means that it requires less substrate to get the enzyme to do it's thing. The enzyme loves it, and it binds tightly. A high Km value means you need a lot of substrate to get the enzyme to act on it. Enzyme feels kinda meh about the substrate in this case.
Why does Vmax decrease?
Vmax decreases because the inhibitor is preventing the product from forming and being released from the enzyme. Km decreases because the inhibitor is effectively increasing the enzyme's affinity for its substrate - so much so that, as mentioned, it is not able to release the substrate. Hope this helps. Upvote.
What does high Km mean?
The enzyme loves it, and it binds tightly. A high Km value means you need a lot of substrate to get the enzyme to act on it. Enzyme feels kinda meh about the substrate in this case. Vmax is simply the rate at which enzyme's do their thing. This is concentration dependent.
What does Km mean in enzymes?
For your first question, the most simple answer is Km tells you binding affinity. In general, if you have a very high Km value, the binding affinity is small. A low Km means binding is strong. Don't memorize here... realize if Km is small, that means that it requires less substrate to get the enzyme to do it's thing. The enzyme loves it, and it binds tightly. A high Km value means you need a lot of substrate to get the enzyme to act on it. Enzyme feels kinda meh about the substrate in this case.
Why does Km increase with a competitive inhibitor?
The reason Km increases with a competitive inhibitor is because the inhibitor is directly competing with the substrate for a fixed number of active sites on enzymes. Hence, Km increases. If there is a competitive inhibitor you will need more substrate to get the same 1/2 Vmax (this is why Km increases). Vmax decreases with a noncompetitive ...