What is shown on the periodic table?
The periodic table shows the atomic mass and atomic number of each element. The atomic number appears above the symbol for the element and the approximate atomic mass appears below it. The periodic table groups elements according to chemical properties.
How many are listed in the periodic table?
This list contains the 118 elements of chemistry. For chemistry students and teachers: The tabular chart on the right is arranged by Atomic number. The first chemical element is Hydrogen and the last is Ununoctium.
Is the periodic table a list of atoms?
The periodic table lists the elements in order of increasing atomic number. Each element has a symbol, which is one or two letters. The first letter is always capitalized.
What are the 118 elements of the periodic table?
The Periodic Table is made up of 118 Elements....Table of 118 Elements - Their Symbols and Atomic Number.ElementAtomic NumberSymbolCarbon6CNitrogen7NOxygen8OFluorine9F83 more rows
How many elements are there in a periodic table 2022?
This is a list of the 118 chemical elements which have been identified as of 2022. A chemical element, often simply called an element, is a type of atom which has the same number of protons in its atomic nucleus (i.e., the same atomic number, or Z).
What are the 4 types of elements?
The Four Elements. Greek philosophy supposed the Universe to comprise four elements: Fire, Water, Earth, and Air.
How can I learn all 118 elements?
Memorization StrategiesBreak down the table into sections. ... Spread out the memorization process. ... Learn the elements in a song. ... Make nonsense words made from element symbols. ... Use color to learn element groups. ... Use a mnemonic device to help remember the order of the elements.
How many metals are on the periodic table?
There are about 70 metals out of 92 natural elements in the periodic table.
Is Diamond an element?
Diamond is a solid form of the element carbon with its atoms arranged in a crystal structure called diamond cubic. At room temperature and pressure, another solid form of carbon known as graphite is the chemically stable form of carbon, but diamond converts to it extremely slowly.
What is the 200th element?
Polonium-200 atom | Po - PubChem.
Is there an element 119?
Ununennium and unbinilium (elements 119 and 120) are the elements with the lowest atomic numbers that have not yet been synthesized.
How can I remember the first 20 elements?
THIS IS A MNEMONIC FOR THE FIRST 20 ELEMENTS.hi hello little begger boy cry not on fallen nemo naught maggie and silly paul sat close at kolaveri cafe.Hi - Hydrogen - H.HEllo - Helium - He.LIttle- Lithium - Li.BEgger- Beryllium- Be.Boy- Boron - B.Cry- Carbon- C.More items...
Has element 119 been made?
Ununennium and unbinilium (elements 119 and 120) are the elements with the lowest atomic numbers that have not yet been synthesized.
How many elements are there in total?
At present, 118 elements are known to us. All these have different properties. Out of these 118, only 94 are naturally occurring.
How many metals are on the periodic table?
There are about 70 metals out of 92 natural elements in the periodic table.
What are the elements 1 to 30?
Atomic Mass of First 30 ElementsATOMIC NUMBERELEMENTATOMIC MASS1Hydrogen1.0082Helium4.00263Lithium6.944Beryllium9.012226 more rows
What is atomic number?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide...
What is the atomic number and mass number?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly diff...
Can two different elements have the same atomic number?
Atoms from two different elements may have the same neutron count, but never the same proton count. The number of protons is unique to the element...
How do we calculate atomic mass?
Add the mass of protons and neutrons to compute the atomic mass of a single atom of an element. Example: Find the atomic mass of a carbon isotope w...
Why is atomic number important?
Atomic number is called the number of protons in an atom. This number is very important, because it is unique to a given element’s atoms. An elemen...
What is the periodic table?
The periodic table, also known as the periodic table of elements, is a tabular display of the chemical elements, which are arranged by atomic number, electron configuration, and recurring chemical properties. The structure of the table shows periodic trends. The seven rows of the table, called periods, generally have metals on ...
How many categories are there in the periodic table?
The elements of the periodic table shown here are divided into nine categories; six for the metals, and two for nonmetals, and a metalloid category. The nine categories (or sets) correspond to those found in the literature for the applicable part of the periodic table. Different authors may use different categorisation schema depending on the properties of interest.
What is the atomic number plotted against?
Atomic number plotted against atomic radius, excluding the noble gases. Atomic radii vary in a predictable and explainable manner across the periodic table. For instance, the radii generally decrease along each period of the table, from the alkali metals to the noble gases; and increase down each group.
What is the electron configuration of a neutral atom?
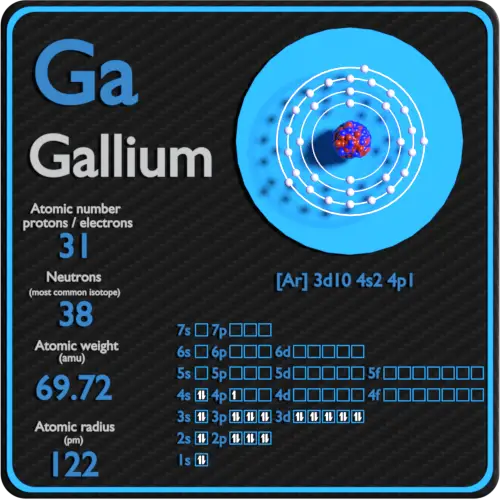
The electron configuration or organisation of electrons orbiting neutral atoms shows a recurring pattern or periodicity. The electrons occupy a series of electron shells (numbered 1, 2, and so on). Each shell consists of one or more subshells (named s, p, d, f and g). As atomic number increases, electrons progressively fill these shells and subshells more or less according to the Madelung rule or energy ordering rule, as shown in the diagram. The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to the start of each new period, these positions being occupied by hydrogen and the alkali metals.
What are metals and nonmetals?
In chronological order, this section discusses metals and nonmetals (and metalloids); categories of elements; groups and periods; and periodic table blocks. While the recognition of metals as solid, fusible and generally malleable substances dates from antiquity, Antoine Lavoisier may have the first to formally distinguish between metals and nonmetals ('non-métalliques') in 1789 with the publication of his 'revolutionary' Elementary Treatise on Chemistry. In 1811, Berzelius referred to nonmetallic elements as metalloids, in reference to their ability to form oxyanions. In 1825, in a revised German edition of his Textbook of Chemistry, he subdivided the metalloids into three classes. These were: constantly gaseous 'gazolyta' (hydrogen, nitrogen, oxygen); real metalloids (sulfur, phosphorus, carbon, boron, silicon); and salt-forming 'halogenia' (fluorine, chlorine, bromine, iodine). Only recently, since the mid-20th century, has the term metalloid been widely used to refer to elements with intermediate or borderline properties between metals and nonmetals. Mendeleev published his periodic table in 1869, along with references to groups of families of elements, and rows or periods of his periodic table. At the same time, Hinrichs wrote that simple lines could be drawn on a periodic table in order to delimit properties of interest, such as elements having metallic lustre (in contrast to those not having such lustre). Charles Janet, in 1928, appears to have been the first to refer to the periodic table's blocks.
How many electrons are in neon?
The electron configuration for neon, for example, is 1s 2 2s 2 2p 6. With an atomic number of ten, neon has two electrons in the first shell, and eight electrons in the second shell; there are two electrons in the s subshell and six in the p subshell. In periodic table terms, the first time an electron occupies a new shell corresponds to ...
What are the columns of periodic table called?
The seven rows of the table, called periods, generally have metals on the left and nonmetals on the right. The columns, called groups , contain elements with similar chemical behaviours.
How many elements are in the periodic table?
The table below consists of 118 elements of the periodic table, sorted by atomic number, atomic weight, symbols, density, discovered year and the group.
Who created the modern periodic table?
The modern periodic table is based on the modern periodic law put forward by the English physicist Henry Moseley, which states that “the properties of elements are periodic functions of their atomic numbers”.
What is the atomic number of an element?
The atomic number of an element is equal to the total number of protons in the nucleus of the atoms of that element. The atomic number can provide insight into the electronic configuration of the element. For example, carbon has an electron configuration of [He] 2s 2 2p 2, since its atomic number is 6.
What is the number of protons in the nucleus called?
The number of protons in the nucleus is called the atomic number. The atomic number of each element is unique.
Why is the atomic number of each element unique?
While the atomic number always stays the same some elements have atoms with different atomic mass numbers. This is because some elements have a different number of neutrons in the nucleus.
How to find the mass of an element?
The number of protons and the number of neutrons shall determine the mass number of an element. Since the isotopes of an element have slightly different mass numbers, it calculates the atomic mass by obtaining the mean of the mass numbers for its isotopes.
How can periodic trends be observed?
Periodic trends in the properties of the elements can be observed down the groups and across the periods of the modern periodic table. Every chemical element has a specific atomic number, which provides insight into the number of protons present within its nucleus.
What are the horizontal rows on the periodic table called?
The horizontal rows on the periodic table are known as Periods.
How many energy shells does period 2 have?
All the elements of period 2 have two energy shells (or orbits).
Why are period 4 and period 5 called long periods?
Period 4 and period 5 are given the name long periods of the periodic table because there are 18 elements in these periods. The elements of the long periods are shown in tables below.
How many periods are there in 2021?
Periods in Periodic table: The periods are the horizontal rows on a Periodic table. There are 7 horizontal rows on Periodic table. Hence there are total 7 periods on the Periodic table.
Why is period 2 called a short period?
Period 2 and period 3 of the periodic table are named as the short period because there are 8 elements in these periods.
Which period has the longest period?
Period 6 and 7: Longest period. Period 5 and period 6 are named as longest periods of the periodic table because there are 32 elements in these periods. The elements of the longest periods are shown in tables below.
Is the Interactive Periodic Table free?
Checkout Interactive Periodic table and download it’s high resolution image now ( It’s FREE)
What are Periodic trends?
You feel hot in summer and cold in winter. That means the climate or weather changes throughout the year starting from January to December.
What is the change in properties of elements down the groups (from top to bottom) and across the periods (from left?
Periodic Trends: The change in properties of elements down the groups (from top to bottom) and across the periods (from left to right) in the Periodic table is known as Periodic trends.
What is the non metallic character?
Non metallic character trend in periodic table is exactly opposite to the metallic character trend. Because nonmetals have exactly the opposite property than that of the metals. Nonmetals are electrons gainers (nonmetals accept/gains electrons) You can also remember the non metallic character from the equation below.
What is electron affinity?
Affinity means attraction. Definition: Electron affinity is defined as the amount of energy released when an electron is added in the outermost shell of an isolated gaseous atom.
How many electrons does oxygen have?
That means oxygen has total 8 electrons. So it’s electron arrangement is (2, 6). It has 2 electrons in first orbit and other 6 electrons in second orbit. You can see that oxygen has 6 electrons in its outermost orbit. Thus, it requires 2 electrons to complete the octet. Hence valency of oxygen is 2.
What happens to the value of an element as you move down the period?
As we move across the period (from left to right), the Valency of the elements first increases and then decreases . While moving down the group (from top to bottom), the Valency of elements remains the same.
Can you find every detail of an interactive periodic table?
You can effortlessly find every single detail about the elements from this single Interactive Periodic table.

Summary
The periodic table, also known as the periodic table of the (chemical) elements, is a tabular display of the chemical elements. It is widely used in chemistry, physics, and other sciences, and is generally seen as an icon of chemistry. It is a graphic formulation of the periodic law, which states that the properties of the chemical elements exhibit an approximate periodic dependence on their atomi…
Overview
The periodic table is a 2-dimensional structured table. The elements are placed in table cells, in reading order of ascending atomic number. The table columns are called groups, the rows are called periods. The breaks at the end of each period occur according to a repetition (or periodicity) of physical and chemical properties of the elements.
Periodic trends
As chemical reactions involve the valence electrons, elements with similar outer electron configurations may be expected to react similarly and form compounds with similar proportions of elements in them. Such elements are placed in the same group, and thus there tend to be clear similarities and trends in chemical behaviour as one proceeds down a group. As analogous configurations return …
Classification of elements
Many terms have been used in the literature to describe sets of elements that behave similarly. The group names alkali metal, alkaline earth metal, pnictogen, chalcogen, halogen, and noble gas are acknowledged by IUPAC; the other groups can be referred to by their number, or by their first element (e.g., group 6 is the chromium group). Some divide the p-block elements from groups 13 to …
History
In 1817, German physicist Johann Wolfgang Döbereiner began to formulate one of the earliest attempts to classify the elements. In 1829, he found that he could form some of the elements into groups of three, with the members of each group having related properties. He termed these groups triads. Chlorine, bromine, and iodine formed a triad; as did calcium, strontium, and barium; lithi…
Current questions
Although the modern periodic table is standard today, some variation can be found in period 1 and group 3. Discussion is ongoing about the placements of the relevant elements. The controversy has to do with conflicting understandings of whether chemical or electronic properties should primarily decide periodic table placement, and conflicting views of how the evidence should be used. A similar potential problem has been raised by theoretical investigations of the superheav…
Future extension beyond the seventh period
The most recently named elements – nihonium (113), moscovium (115), tennessine (117), and oganesson (118) – completed the seventh row of the periodic table. Future elements would have to begin an eighth row. These elements may be referred to either by their atomic numbers (e.g. "element 119"), or by the IUPAC systematic element names which directly relate to the atomic …
Alternative periodic tables
The periodic law may be represented in multiple ways, of which the standard periodic table is only one. Within 100 years of the appearance of Mendeleev's table in 1869, Edward G. Mazurs had collected an estimated 700 different published versions of the periodic table. Many forms retain the rectangular structure, including Janet's left-step periodic table (pictured below), and the m…