
The quaternary structure of a protein is the association of several protein chains or subunits into a closely packed arrangement. Each of the subunits has its own primary, secondary, and tertiary structure. The subunits are held together by hydrogen bonds and van der Waals forces between nonpolar side chains.
What does protein quaternary structure mean?
Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex.
What is the quaternary structure of a protein?
The quaternary structure of a protein is the association of several protein chains or subunits into a closely packed arrangement. Each of the subunits has its own primary, secondary, and tertiary structure. The subunits are held together by hydrogen bonds and van der Waals forces between nonpolar side chains.
Do all proteins have to have quaternary structure?
Only those proteins that are made of multiple polypeptide chains have a quaternary structure, while proteins made from a single polypeptide do not. DNA and hemoglobin are examples of proteins that have a quaternary structure.
What do proteins have no quaternary structure?
You cannot have quaternary structure without secondary and tertiary structure. However, you can have proteins that have no quaternary structure, but all proteins have primary, secondary and tertiary structure. Proteins without quaternary structure are those with single polypeptide chains.
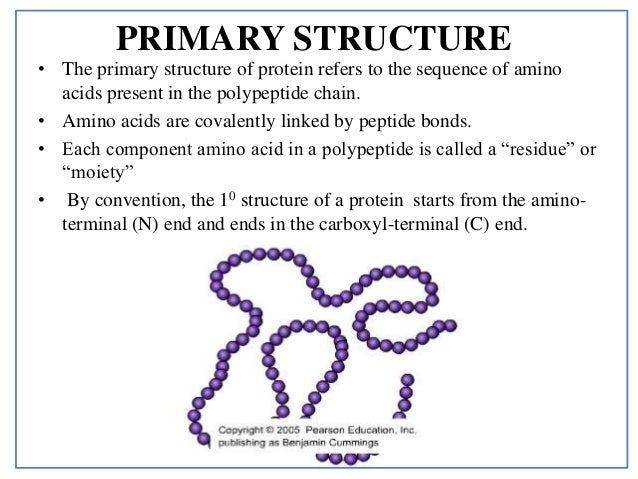
What defines quaternary structure?
Quaternary structure exists in proteins consisting of two or more identical or different polypeptide chains (subunits). These proteins are called oligomers because they have two or more subunits. The quaternary structure describes the manner in which subunits are arranged in the native protein.
What is the function of a quaternary protein structure?
Quaternary structure is an important protein attribute that is closely related to its function. Proteins with quaternary structure are called oligomeric proteins. Oligomeric proteins are involved in various biological processes, such as metabolism, signal transduction, and chromosome replication.
What is an example of a protein with quaternary structure?
We've already encountered one example of a protein with quaternary structure: hemoglobin. As mentioned earlier, hemoglobin carries oxygen in the blood and is made up of four subunits, two each of the α and β types.
Which best describes the quaternary structure of a protein?
Which of the following best describes the quaternary structure of a protein? Explanation: Quaternary structure describes how polypeptide chains fit together to form a complete protein. Quaternary protein structure is held together by hydrophobic interactions, and disulfide bridges.
What is the difference between tertiary and quaternary structure of proteins?
Tertiary structure refers to the configuration of a protein subunit in three-dimensional space, while quaternary structure refers to the relationships of the four subunits of hemoglobin to each other.
What are the 4 levels of a protein structure?
Proteins fold into stable three‐dimensional shapes, or conformations, that are determined by their amino acid sequence. The complete structure of a protein can be described at four different levels of complexity: primary, secondary, tertiary, and quaternary structure.
What is the quaternary structure of a protein quizlet?
The Quaternary structure of the protein is two or more polypeptide chains bonded together, and maintained by the same interactions as the tertiary structure.
What is the 4 levels of organization of proteins explain each?
Answer. The four levels of protein structure are primary, secondary, tertiary, and quaternary structure, which are distinguished from one another by the degree of complexity in the polypeptide chain.
What is the quaternary structure of a protein quizlet?
The Quaternary structure of the protein is two or more polypeptide chains bonded together, and maintained by the same interactions as the tertiary structure.
What is the function of secondary protein structure?
Secondary structure of the proteins can be used to predict the tertiary structure since predicting only with amino acid sequence may not be sufficient. The secondary structure of proteins is determined by the pattern of hydrogen bonding.
How to determine quaternary structure of protein?
Protein quaternary structure can be determined using a variety of experimental techniques that require a sample of protein in a variety of experimental conditions. The experiments often provide an estimate of the mass of the native protein and, together with knowledge of the masses and/or stoichiometry of the subunits, allow the quaternary structure to be predicted with a given accuracy. It is not always possible to obtain a precise determination of the subunit composition for a variety of reasons.
What is quaternary structure?
The quaternary structure refers to the number and arrangement of the protein subunits with respect to one another. Examples of proteins with quaternary structure include hemoglobin, DNA polymerase, and ion channels . Enzymes composed of subunits with diverse functions are sometimes called holoenzymes, in which some parts may be known as regulatory ...
What is the term for a protein that is made from multiple copies of a polypeptide?
When multiple copies of a polypeptide encoded by a gene form a quaternary complex, this protein structure is referred to as a multimer . When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. In such a case, the phenomenon is referred to as intragenic complementation (also called inter-allelic complementation). Intragenic complementation appears to be common and has been studied in many different genes in a variety of organisms including the fungi Neurospora crassa, Saccharomyces cerevisiae and Schizosaccharomyces pombe; the bacterium Salmonella typhimurium; the virus bacteriophage T4, an RNA virus, and humans. The intermolecular forces likely responsible for self-recognition and multimer formation were discussed by Jehle.
How many subunits are in an oligomeric complex?
Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
How to determine the number of subunits in a protein complex?
The number of subunits in a protein complex can often be determined by measuring the hydrodynamic molecular volume or mass of the intact complex , which requires native solution conditions.
What is dual polarisation interferometry?
Dual polarisation interferometry (meas ures the size and the density of the complex)
Which molecular machine is composed of multiples of 60 proteins?
Although complexes higher than octamers are rarely observed for most proteins, there are some important exceptions. Viral capsids are often composed of multiples of 60 proteins. Several molecular machines are also found in the cell, such as the proteasome (four heptameric rings = 28 subunits), the transcription complex and the spliceosome. The ribosome is probably the largest molecular machine, and is composed of many RNA and protein molecules.
How to determine quaternary structure?
The quaternary structure is usually determined by X-ray crystallography, as described previously. However, when crystallographic data were difficult or impossible to gather, electron microscopy had provided some clues to quaternary structure.
How are quaternary structures held together?
Quaternary structure is held together by noncovalent bonds between complementary surface hydrophobic and hydrophilic regions on the polypeptide subunits. Additionally, acidic and basic side chains can form salt linkages. Since the same weak forces that stabilize tertiary structure are involved in stabilizing quaternary structure, the subunits can be dissociated from each other. It is also possible to have covalent stabilization by inter-chain disulfide bonds.
Why are oligomers called quaternary structures?
These proteins are called oligomers because they have two or more subunits. The quaternary structure describes the manner in which subunits are arranged in the native protein. Subunits are held together by noncovalent forces; as a result, oligomeric proteins can undergo rapid conformational changes that affect biological activity. Oligomeric proteins include the hemoglobins (Chapter 28), allosteric enzymes (Chapter 7) responsible for the regulation of metabolism, and contractile proteins such as actin and tubulin (Chapter 21 ).
What is the structure of the 30S and 50S subunits?
Subunit structures of both the 30S and 50S subunits at resolutions higher than the intact 70S have been achieved. Two research groups have studied the crystal structure of the 30S subunit.70,71 The 16S rRNA of the 30S subunit consists of over 50 double-stranded helices connected by irregular single-stranded regions; thus the RNA structure is a three-dimensional arrangement of helices. The packing of the helices determines the overall structure of the four rRNA domains. The ribosomal proteins generally have a globular domain that is on the surface of the 30S, with long extensions that reach into the 30S, making extensive interactions with the rRNA and stabilizing the overall ribosome structure. The A and P tRNA binding sites are each composed of rRNA from two different domains of the 16S rRNA. These two domains have been observed to move relative to one another in cryoelectron microscopy studies, and this motion is almost certainly involved in the motions of tRNAs and mRNA during translation. The three tRNAs bind their anticodon regions in the RNA-rich region that forms a groove in the 30S subunit.60 Several classes of antibiotics including major clinical classes such as spectinomycin, tetracyclines, and aminoglycosides bind to the 30S as their site of action.72,73
What is heteromeric vs homomeric?
Heteromeric: composed of different subunits, each produced by a different gene. •. Homomeric: composed of the same monomer unit, produced by the same gene. Quaternary structure is held together by noncovalent bonds between complementary surface hydrophobic and hydrophilic regions on the polypeptide subunits.
What are oligomeric proteins?
Oligomeric proteins include the hemoglobins, allosteric enzymes responsible for the regulation of metabolism, contractile proteins such as actin and tubulin, and cell membrane proteins. Several proteins inserted into the membrane participate in the transport of metabolites and ions.
What is the PQS server?
The Protein Quaternary Structure (PQS) server42,43 performs such analyses for all x-ray structures deposited in the PDB.
What is the quaternary structure of a protein?
The quaternary structure of a protein refers to an arrangement of folded subunits of that protein in its primary, secondary, and tertiary structure as a multi-subunit complex. All such subunits are held together by hydrogen bonding and Vander Waal’s forces of attraction.
Why is the quaternary state important?
The quaternary state is inconsequential and is vital to the enzymatic activity in some of the oligomeric enzymes, which occurs because the amino acid residues in the specific active site come from more than one subunit.
Which interaction provides the association of the subunits into higher aggregates?
The interaction that provides the association of the subunits into higher aggregates is the same as those that cause the folding of the proteins. The entropy factors are important in the formation of the quaternary structure.
What is the function of symmetry in DNA?
The functional role of the symmetry is found during the binding of the dimeric repressor proteins to the palindromic sequences of the double stranded DNA. The twofold symmetry of the DNA-binding proteins is necessary for binding to the double stranded DNA.
What is the stoichiometry of a symmetrical oligomeric molecule?
The stoichiometry of the symmetrical oligomeric molecule is related to the number of subunits, except for the reaction that takes part close to the symmetry element. For example, the binding of diphosphoglycerate to the β subunits of the hemoglobin. The single molecule of the diphosphoglycerate flanks the twofold rotation axis between the two subunits because of the location of the binding site. A single binding site is present per two β-subunits and per hemoglobin tetramer.
Which amino acid clusters on the inside of a protein?
Also important to tertiary structure are hydrophobic interactions, in which amino acids with nonpolar, hydrophobic R groups cluster together on the inside of the protein, leaving hydrophilic amino acids on the outside to interact with surrounding water molecules.
What are the four levels of protein structure?
To understand how a protein gets its final shape or conformation, we need to understand the four levels of protein structure: primary, secondary, tertiary, and quaternary.
How are the amino acids in insulin connected?
Image of insulin. Insulin consists of an A chain and a B chain. They are connected to one another by disulfide bonds (sulfur-sulfur bonds between cysteines). The A chain also contains an internal disulfide bond. The amino acids that make up each chain of insulin are represented as connected circles, each with the three-letter abbreviation of the amino acid's name.
How many polypeptide chains are there in insulin?
For example, the hormone insulin has two polypeptide chains, A and B, shown in diagram below. (The insulin molecule shown here is cow insulin, although its structure is similar to that of human insulin.) Each chain has its own set of amino acids, assembled in a particular order.
What are the different order of proteins?
Orders of protein structure: primary, secondary, tertiary, and quaternary. Alpha helix and beta pleated sheet.
Which amino acids have large ring structures in their R groups?
Similarly, amino acids such as tryptophan, tyrosine, and phenylalanine, which have large ring structures in their R groups, are often found in β pleated sheets, perhaps because the β pleated sheet structure provides plenty of space for the side chains.
How is the sequence of a protein determined?
The sequence of a protein is determined by the DNA of the gene that encodes the protein (or that encodes a portion of the protein, for multi-subunit proteins). A change in the gene's DNA sequence may lead to a change in the amino acid sequence of the protein. Even changing just one amino acid in a protein’s sequence can affect ...

Overview
Protein quaternary structure is the fourth (and highest) classification level of protein structure. Protein quaternary structure refers to the structure of proteins which are themselves composed of two or more smaller protein chains (also referred to as subunits). Protein quaternary structure describes the number and arrangement of multiple folded protein subunits in a multi-subunit complex. It in…
Description and examples
Many proteins are actually assemblies of multiple polypeptide chains. The quaternary structure refers to the number and arrangement of the protein subunits with respect to one another. Examples of proteins with quaternary structure include hemoglobin, DNA polymerase, ribosomes, antibodies, and ion channels.
Enzymes composed of subunits with diverse functions are sometimes called holoenzymes, in wh…
Nomenclature
The number of subunits in an oligomeric complex is described using names that end in -mer (Greek for "part, subunit"). Formal and Greco-Latinate names are generally used for the first ten types and can be used for up to twenty subunits, whereas higher order complexes are usually described by the number of subunits, followed by -meric.
Structure Determination
Protein quaternary structure can be determined using a variety of experimental techniques that require a sample of protein in a variety of experimental conditions. The experiments often provide an estimate of the mass of the native protein and, together with knowledge of the masses and/or stoichiometry of the subunits, allow the quaternary structure to be predicted with a given accuracy. It is not always possible to obtain a precise determination of the subunit composition …
Structure Prediction
Some bioinformatics methods have been developed for predicting the quaternary structural attributes of proteins based on their sequence information by using various modes of pseudo amino acid composition.
Protein folding prediction programs used to predict protein tertiary structure have also been expanding to better predict protein quaternary structure. One such development is AlphaFold-M…
Role in Cell Signaling
Protein quaternary structure also plays an important role in certain cell signaling pathways. The G-protein coupled receptor pathway involves a heterotrimeric protein known as a G-protein. G-proteins contain three distinct subunits known as the G-alpha, G-beta, and G-gamma subunits. When the G-protein is activated, it binds to the G-protein coupled receptor protein and the cell signaling pathway is initiated. Another example is the receptor tyrosine kinase (RTK) pathway, w…
Protein–protein interactions
Proteins are capable of forming very tight complexes. For example, ribonuclease inhibitor binds to ribonuclease A with a roughly 20 fM dissociation constant. Other proteins have evolved to bind specifically to unusual moieties on another protein, e.g., biotin groups (avidin), phosphorylated tyrosines (SH2 domains) or proline-rich segments (SH3 domains). Protein-protein interactions can be engineered to favor certain oligomerization states.
Intragenic complementation
When multiple copies of a polypeptide encoded by a gene form a quaternary complex, this protein structure is referred to as a multimer. When a multimer is formed from polypeptides produced by two different mutant alleles of a particular gene, the mixed multimer may exhibit greater functional activity than the unmixed multimers formed by each of the mutants alone. In such a case, the phenomenon is referred to as intragenic complementation (also called inter-allelic complementat…