Definition of electric double layer : a region existing at the boundary of two phases and assumed to consist of two oppositely charged layers (such as a layer of negative ions adsorbed on colloidal
Colloid
A colloid, in chemistry, is a substance in which one substance of microscopically dispersed insoluble particles is suspended throughout another substance.
What is a double layer in chemistry?
(Show more) electrical double layer, region of molecular dimension at the boundary of two substances across which an electrical field exists. The substances must each contain electrically charged particles, such as electrons, ions, or molecules with a separation of electrical charges (polar molecules).
What is meant by double layer in electrolysis?
electric double layer. noun. : a region existing at the boundary of two phases and assumed to consist of two oppositely charged layers (such as a layer of negative ions adsorbed on colloidal particles that attracts a layer of positive ions in the surrounding electrolytic solution) — called also Helmholtz double layer.
What happens when a double layer of charge is applied?
The sheets of charge cause a strong electric field and a correspondingly sharp change in voltage ( electrical potential) across the double layer. Ions and electrons which enter the double layer are accelerated, decelerated, or reflected by the electric field.
What is the electrical potential of the electric double layer?
The electrical potential within the electric double layer has a maximum value on the particle surface (Stern layer). The potential drops with the increase of distance from the surface and reaches 0 at the boundary of the electric double layer.
What is meant by electrical double layer and zeta potential?
The zeta potential gives an indication of the potential stability of the colloidal system. Electrical double layer: There is a region around each particle where the particle charge attracts the free ions to form an electrical cloud called the electrical double layer.
What is electrical double layer capacitance?
Electric double layer capacitor (EDLC) [1,2] is the electric energy storage system based on charge–discharge process (electrosorption) in an electric double layer on porous electrodes, which are used as memory back-up devices because of their high cycle efficiencies and their long life-cycles.
What is double layer effect?
An electric double layer is a phenomenon that plays a fundamental role in the mechanism of the electrostatic stabilization of colloids. Colloidal particles gain negative electric charge when negatively charged ions of the dispersion medium are adsorbed on the particle surface.
What is double layer charging current?
The double layer charging current arises because of the capacitive-like nature of the electrode/electrolyte interface. The current (I) through a capacitor is given by the product of the capacitance (C) and the rate of change in voltage (dV/dt): I (Amp) = C (Faraday) x dV/dt (Volt/sec).
How do you find the capacitance of a double layer?
Take the current width of the CV (in amps, pick a point in the CV where there is no faradaic process occurring) and divide that by the scan rate of the CV (V/s). Amps is Coulombs/s divided by Volts/s = Coulombs / Volt which is Farrads.
How is double layer capacitance formed?
An electrical double layer exists at the interface between an electrode and its surrounding electrolyte. This double layer is formed as ions from the solution "stick to" the surface of the electrode. Charges in the electrode are separated from the charges of these ions.
What is electrode double layer capacitor?
An Electrochemical Double Layer Capacitor (EDLC) System is an energy storage system based on electrostatic effects that occur between two carbon electrodes with high specific surface areas per volume, e.g. activated carbons. The electrodes are immersed in an electrolyte, and a separator between the electrodes is used.
Which model of double layer capacitor is called as constant capacity model?
But in solution, due to thermal motion of liquid molecules such a rigid array of charges at an interface may not exist. 2) Helmholtz parallel plate condenser model predicts a constant capacity (C) [that is, one which does not change with potential] for the electrical double layer.
What is the electrical double layer?
Electrical double layer, region of molecular dimension at the boundary of two substances across which an electrical field exists. The substances must each contain electrically charged particles, such as electrons, ions, or molecules with a separation of electrical charges (polar molecules). In the electrical double layer, oppositely charged ...
What causes an electrical field to be established across the interface?
The electrostatic attraction between the two opposite and separated charges causes an electrical field to be established across the interface. The electrical field generated within an electrical double layer has a major influence on physical and chemical processes that occur at phaseboundaries.
What is the electric field?
electric field. Electric field, an electric property associated with each point in space when charge is present in any form. The magnitude and direction of the electric field are expressed by the value of E, called electric field strength or electric field intensity or simply the electric field. Knowledge of the value….
What is the electrical double layer?
The electrical double layer at the oil/water interface is a heterogeneous interfacial region that separates two bulk phases of polarized media and maintains a spatial separation of charges. Electrical double layers at such interfaces determine the kinetics of charge transfer across phase boundaries, stability and electrokinetic properties of lyophobic colloids, mechanisms of phase transfer or interfacial catalysis, charge separation in natural and artificial photosynthesis, and heterogeneous enzymatic catalysis [ 8, 59-70 ].
What is double layer capacitor?
An electrical double-layer capacitor is a capacitor with a high capacity, higher than for usual capacitors; however, it has lower voltage limits. It fills the gap between the rechargeable batteries and the electrolytic capacitors. They have the advantage over batteries in that they can accept and produce electrical charge much faster and larger than that a battery can handle, and they have the advantage over the electrolytic capacitor in they have 10–100 times higher energy density. Some of the applications of the double-layer capacitors are regenerative braking system, where it requires rapid charging and discharging cycles. From the name of the double-layer capacitor, they use electrostatic double-layer capacitance, which uses carbon electrodes that achieves the separation charge at the interface between the electrolyte and the electrode.
What is polarizable interface?
The interface between two immiscible electrolyte solutions (ITIES) can be either polarizable or non-polarizable, depending on permeability to charged particles. If the interface is relatively impermeable it is called polarizable. Otherwise it is called non-polarizable or reversible. Planck [ 71, 72] introduced a rigorous thermodynamic concept called a completely polarizable interface, defined as " an interface whose state is completely determined by the charge that has passed through it beginning from a given instant. " Planck's definition assumes that direct current cannot flow through the interface [71]. There can only be the transitive current in the boundary layer. However, the definition says nothing about how charged particles from different phases are distributed in this transitional layer. The distribution of particles in the boundary layer can have any configuration and the tales of this distribution for different particles can even overlap in the boundary layer ( Fig. 19 a,c ). At a completely polarizable interface charge transfer between bulk phases becomes impossible.
What is the significance of the EDL structure?
Electrical double layer structure (edl) and potential of zero charge are the fundamental characteristics of the electrode/electrolyte interface, determining the charge and mass transfer kinetics.1–12 Edl structure determines the properties of electrical double layer capacitors and hybrid supercapacitors, various batteries, rate of faradic gas adsorption/evolution, and reionization as well as corrosion. Electrical double layer structure plays an important role also in electrosynthesis, electroanalysis and even in electrochemical photovoltaic cells. 13–15
What is the layer of ions that surrounds a particle suspended in an electrolyte solution?
In the electrical double layer surrounding a particle suspended in an electrolyte solution, a layer of ions is attracted to the surface of the particle due to chemical and electrochemical reactions.
What are the techniques used to determine the structure of a single crystal surface?
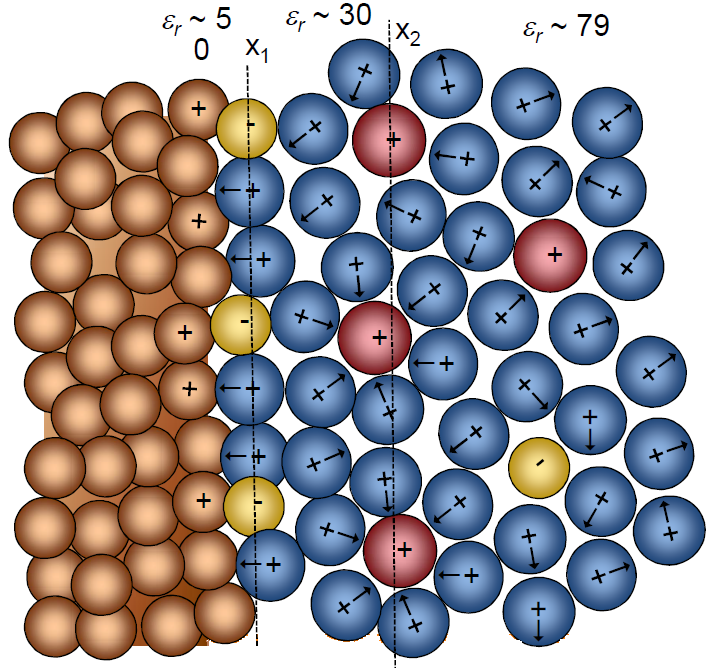
The most widely used techniques for the determination of the structure of single-crystal surfaces are low-energy electron diffraction (LEED), high-resolution transmission electron microscopy (HRTEM), in situ Fourier transmission infra red (FTIR) and Raman, second harmonic generation, scanning tunneling microscopy (STM), atomic force microscopy (AFM), surface X-ray scattering (SXRS), and other methods. 3–5,7–9 Auger electron spectroscopy and HRTEM (combined with energy-dispersive X-ray spectroscopy, selected area electron diffraction, electron energy loss spectroscopy) and focused ion beam—time of flight—secondary ion mass spectroscopy are used to characterize the chemical composition or cleanliness of surface prepared using novel surface preparation methods (flame annealing, electrochemical polishing, ultra high vacuum (UHV), and atomic layer deposition). To the first approximation, the planes with low Miller indexes for some metals (Ag, Bi, Sb, Cd, Pt) reflect the symmetry of the bulk structure, and they have lower surface energy than the high-index planes. 3–5 However, LEED, AFM, STM, and SXRS studies revealed two important features of atomic surface structure established for some Au planes: relaxation and reconstruction, 5,7–10 depending on electrolyte composition, pH, and electrochemical polarization.
Why do double layers exhibit significant rates of self-discharge?
Electrical double layers (EDLs) exhibit significant rates of self-discharge mainly as a consequence of the ease with which the charge on the electrode can be affected by impurity reactions.
What is the double layer of charge?
A double layer ( DL, also called an electrical double layer, EDL) is a structure that appears on the surface of an object when it is exposed to a fluid. The object might be a solid particle, a gas bubble, a liquid droplet, or a porous body. The DL refers to two parallel layers of charge surrounding the object. The first layer, the surface charge (either positive or negative), consists of ions adsorbed onto the object due to chemical interactions. The second layer is composed of ions attracted to the surface charge via the Coulomb force, electrically screening the first layer. This second layer is loosely associated with the object. It is made of free ions that move in the fluid under the influence of electric attraction and thermal motion rather than being firmly anchored. It is thus called the "diffuse layer".
What is EDL in physics?
The electrical double layer ( EDL) is the result of the variation of electric potential near a surface, and has a significant influence on the behaviour of colloids and other surfaces in contact with solutions or solid-state fast ion conductors .
How is an EDL formed?
The formation of electrical double layer (EDL) has been traditionally assumed to be entirely dominated by ion adsorption and redistribution. With considering the fact that the contact electrification between solid-solid is dominated by electron transfer, it is suggested by Wang that the EDL is formed by a two-step process. In the first step, when the molecules in the solution first approach a virgin surface that has no pre-existing surface charges, it may be possible that the atoms/molecules in the solution directly interact with the atoms on the solid surface to form strong overlap of electron clouds. Electron transfer occurs first to make the “neutral” atoms on solid surface become charged, i.e., the formation of ions. In the second step, if there are ions existing in the liquid, such as H+ and OH-, the loosely distributed negative ions in the solution would be attracted to migrate toward the surface bonded ions due to electrostatic interactions, forming an EDL. Both electron transfer and ion transfer co-exist at liquid-solid interface.
Why is the thin DL model valid for most aqueous systems?
The thin DL model is valid for most aqueous systems because the Debye length is only a few nanometers in such cases. It breaks down only for nano-colloids in solution with ionic strengths close to water.
What is the boundary between an electrolyte and an electronic conductor?
When an electronic conductor is brought in contact with a solid or liquid ionic conductor (electrolyte), a common boundary ( interface) among the two phases appears. Hermann von Helmholtz was the first to realize that charged electrodes immersed in electrolyte solutions repel the co-ions of the charge while attracting counterions to their surfaces. Two layers of opposite polarity form at the interface between electrode and electrolyte. In 1853 he showed that an electrical double layer (DL) is essentially a molecular dielectric and stores charge electrostatically. Below the electrolyte's decomposition voltage, the stored charge is linearly dependent on the voltage applied.
What is the theory of a flat surface and a symmetrical electrolyte?
The theory for a flat surface and a symmetrical electrolyte is usually referred to as the Gouy-Chapman theory . It yields a simple relationship between electric charge in the diffuse layer σ d and the Stern potential Ψ d:
Which model fails for highly charged DLs?
The Gouy-Chapman model fails for highly charged DLs. In 1924 Otto Stern suggested combining the Helmholtz model with the Gouy-Chapman model: In Stern's model, some ions adhere to the electrode as suggested by Helmholtz, giving an internal Stern layer, while some form a Gouy-Chapman diffuse layer.
What is meant by electrical double layer and zeta potential?
Zeta Potential is the electric potential at the shear plane of a particle. Particles within a colloidal dispersion carry charges that contribute to the net charge of a particle. These along with the ions in the stern layer form the electrical double layer at the particle-liquid interface.
What is double layer effect?
The formation of double layers is exploited in every electrochemical capacitor to store electrical energy. It is at this interface that the double layer effect occurs. When a voltage is applied to the capacitor, two layers of polarized ions are generated at the electrode interfaces.
What do you mean by Helmholtz electrical double layer?
Helmholtz double layer (HDL) refers to the structural representation of the accumulation of electrical charges present at the boundary of an electrode and electrolyte when they are in contact with each other. HDL is most readily identifiable in fluid-based mixture systems, such as paints used for corrosion prevention.
What is zeta potential give an example?
Zeta potential is defined as the potential difference between the dispersion medium and the stationary layer of fluid attached to the particle and it is measured by a zeta potential analyzer.
Why is electrical double layer important?
An electric double layer is a phenomenon that plays a fundamental role in the mechanism of the electrostatic stabilization of colloids. Colloidal particles gain negative electric charge when negatively charged ions of the dispersion medium are adsorbed on the particle surface.
What is double layer repulsion?
Double layer forces occur between charged objects across liquids, typically water. For two similarly charged objects, this force is repulsive and decays exponentially at larger distances, see figure. For unequally charged objects and eventually at shorted distances, these forces may also be attractive.
What is double layer thickness?
Diffuse double layer (DDL) is an ionic structure that describes the variation of electric potential near a charged surface, such as clay, and behaves as a capacitor. The thickness of the DDL is <10 −6 cm (Pamukcu, 1997). Models: The Helmholtz, Gouy−Chapman, and Gouy−Chapman−Stern models describe the structure of a DDL.
What is double layer?
A double layer is a structure in a plasma and consists of two parallel layers with opposite electrical charge. The sheets of charge cause a strong electric field and a correspondingly sharp change in voltage ( electrical potential) across the double layer. Ions and electrons which enter the double layer are accelerated, decelerated, ...
What is a current carrying double layer?
Current carrying double layers may be generated by current-driven plasma instabilities which amplify variations of the plasma density. Current-free double layers form on the interface between two plasma regions with different characteristics, and their associated electric field maintains a balance between the penetration of electrons in either direction (so that the net current is low).
How do double layers form?
One example is the Buneman instability which occurs when the streaming velocity of the electrons (basically the current density divided by the electron density) exceeds the electron thermal velocity of the plasma. Double layers (and other phase space structures) are often formed in the non-linear phase of the instability. One way of viewing the Buneman instability is to describe what happens when the current (in the form of a zero temperature electron beam) has to pass through a region of decreased ion density. In order to prevent charge from accumulating, the current in the system must be the same everywhere (in this 1D model). The electron density also has to be close to the ion density (quasineutrality), so there is also a dip in electron density. The electrons must therefore be accelerated into the density cavity, to maintain the same current density with a lower density of charge carriers. This implies that the density cavity is at a high electrical potential. As a consequence, the ions are accelerated out of the cavity, amplifying the density perturbation. Then there is the situation of a double-double layer, of which one side will most likely be convected away by the plasma, leaving a regular double layer. This is the process in which double layers are produced along planetary magnetic field lines in so-called Birkeland currents.
How do current free double layers form?
We explain how they form (neglecting the ions which are considered solely as a neutralizing background). Consider a plasma divided into two regions by a plane, which has a higher electron temperature on one side than on the other (the same analysis can also be done for different densities). This means that the electrons on one side of the interface have a greater thermal velocity. The electrons may stream freely in either direction, and the flux of electrons from the hot plasma to the cold plasma will be greater than the flux of the electrons from the cold plasma to the hot plasma, because the electrons from the hot side have a greater average speed. Because many more electrons enter the cold plasma than exit it, part of the cold region becomes negatively charged. The hot plasma, conversely, becomes positively charged. Therefore, an electric field builds up, which starts to accelerate electrons towards the hot region, reducing the net flux. In the end, the electric field builds up until the fluxes of electrons in either direction are equal, and further charge build up in the two plasmas is prevented. The potential drop is in fact exactly equal to the difference in thermal potential between the two plasma regions in this case, so such a double layer is a marginally strong double layer.
What happens to electrons in a double layer?
Ions and electrons which enter the double layer are accelerated, decelerated, or reflected by the electric field. In general, double layers (which may be curved rather than flat) separate regions of plasma with quite different characteristics.
How stable are double layers?
Stability: Double layers in laboratory plasmas may be stable or unstable depending on the parameter regime. Various types of instabilities may occur, often arising due to the formation of beams of ions and electrons. Unstable double layers are noisy in the sense that they produce oscillations across a wide frequency band. A lack of plasma stability may also lead to a dramatic change in configuration often referred to as an explosion (and hence exploding double layer ). In one example, the region enclosed in the double layer rapidly expands and evolves. An explosion of this type was first discovered in mercury rectifiers used in high-power direct-current transmission lines, where the voltage drop across the device was seen to increase by several orders of magnitude. Double layers may also drift, usually in the direction of the emitted electron beam, and in this respect are natural analogues to the smooth bore magnetron.) (not to be confused with a unit of magnetic moment, the Bohr magnetron, which is created by the “classical circular motion” of an electron around a proton).
How are double layers classified?
Double layers may be classified in the following ways: Weak and strong double layers. The strength of a double layer is expressed as the ratio of the potential drop in comparison with the plasma’s equivalent thermal potential, or in comparison with the rest mass energy of the electrons.
