The oxygen–hemoglobin dissociation curve, also called the oxyhemoglobin
Hemoglobin
Hemoglobin; also spelled haemoglobin and abbreviated Hb or Hgb, is the iron-containing oxygen-transport metalloprotein in the red blood cells of all vertebrates (with the exception of the fish family Channichthyidae) as well as the tissues of some invertebrates. Hemoglobin in the blood …
What do you mean by O2 dissociation curve?
The oxygen dissociation curve is the expression of the relationship between the partial pressure of oxygen and oxygen saturation of haemoglobin.
What is oxygen dissociation curve Class 11?
The oxygen dissociation curve is a graph showing the percentage saturation of oxyhaemoglobin at various partial pressures of oxygen. The curve shows the equilibrium of oxyhaemoglobin and haemoglobin at various partial pressures. In the lungs, the partial pressure of oxygen is high.
What is oxygen dissociation curve Byjus?
When the oxygen saturation percentage is plotted against the oxygen partial pressure, or pO2, an S-shaped or sigmoid curve is obtained. It is called the oxygen dissociation curve.
What is oxygen dissociation curve PDF?
An oxyhaemoglobin dissociation curve (ODC) quantifies the most important function of red blood cells and that is the affinity for oxygen and its delivery to the tissues. Oxygen affinity for haemoglobin plays a critical role in the delivery of oxygen to the tissues and is changed by shifting to the left or right.
What factors affect oxygen dissociation curve?
Classically the factors recognised to influence the oxygen dissociation curve (ODC) include the local prevailing CO2 partial pressure (PCO2), pH and temperature. The curve is shifted to the right (i.e. lower saturation for a given PO2) by higher PCO2, greater acidity (lower pH) and higher temperature.
How is respiration regulated Class 11 bio?
In order to control the rate and depth of breathing, the respiratory center gets input from chemoreceptors, mechanoreceptors, the cerebral cortex, and the hypothalamus.
What is the shape of hemoglobin oxygen dissociation curve?
The shape of the oxygen dissociation curve of Hb is sigmoidal, whereas that of other oxygen-carrying molecules (such as Myoglobin) is hyperbolic.
What causes the oxygen dissociation curve to shift to the right?
The right shift in the oxygen dissociation curve is due to an increase in partial pressure of carbon dioxide or a decrease in the pH.
Why is the oxygen dissociation curve sigmoid?
The oxygen dissociation curve shown in Figure 71-2 is sigmoid shaped because of heme–heme interactions. Mutations that affect the heme–heme interaction, the Bohr effect, or the deoxyhemoglobin-2,3-DPG interaction can change the shape or position of the oxygen dissociation curve. Mutations affecting the α 1 β 2 subunit contact point can alter heme–heme interaction by causing the deoxyhemoglobin conformation to be less table. These mutations result in increased stability of the oxyhemoglobin conformation and increased oxygen affinity (e.g. Hb Kempsey (β99 Asp → Asn)). Alternatively, the oxyhemoglobin conformation can be destabilized by mutations affecting the α94β102 contact point, resulting in decreased oxygen affinity (e.g. Hb Kansas (β102 Asn → Thr)). Substitutions at the COOH-terminal ends of globin chains can lead to instability of deoxyhemoglobin conformations and increased oxygen affinity (e.g. Hb Bethesda (β145 Tyr → His)) as well as a reduction in the Bohr effect. 2,3-DPG binds to residues β1, 2, 82, and 143 in the deoxygenated form. Substitutions altering these residues tend to have increased oxygen affinity (e.g. Hb F (γ-globin has a serine for histidine substitution at position 143)).
How does oxygen dissociation work?
The oxygen dissociation curve is a graph with oxygen partial pressure along the horizontal axis and oxygen saturation on the vertical axis, which shows an S-shaped relationship. Oxygen and carbon dioxide are transported in the blood as a result of changes in blood partial pressures ( Figure 5.1 ). Most oxygen is taken into the hemoglobin in red blood cells, although trace levels of oxygen exist in the dissolved form. The oxygen content is about 20 ml, when is hemoglobin at 15 g per 100 ml of blood is 100% saturated with oxygen. It has the characteristic advantages of taking in oxygen via the lungs and dissociation of oxygen in organs. With increased carbon dioxide excretion, increased hydrogen ion (proton, H +) concentration (fall in pH) and increased partial temperature, the oxygen dissociation curve is shifted to the right, promoting oxygen dissociation. At this time, the affinity of hemoglobin for oxygen ( P50) becomes large.
How does acidosis affect oxygen delivery?
Acidosis causes a right shift in the oxyhaemoglobin dissociation curve (the Bohr effect, see p. 90) and this facilitates oxygen delivery to tissues. An increase in hydrogen ion concentration decreases erythrocyte 2,3-diphosphoglycerate (2,3-DPG) concentrations through effects on both synthesis and breakdown; this causes a left shift in the curve, but, whereas the Bohr effect is immediate, the fall in 2,3-DPG takes place over a matter of hours. The reverse is also true; so, if hydrogen ion concentration is restored to normal rapidly, oxygen delivery will be compromised until 2,3-DPG concentrations are restored to normal. This is a potential hazard if an attempt is made to correct an acidosis rapidly by the intravenous infusion of bicarbonate.
What does it mean when the hemoglobin curve is left?
A shift to the left indicates increased affinity and so an increased tendency for haemoglobin to take up and retain oxygen.
What is the Bohr effect?
This effect, originally described by C. Bohr, 63 is mainly a result of changes in pH, although CO 2 itself has some direct effect. The Bohr effect is given a numeric value, Δlog p50 O 2 /ΔpH, where Δlog p50 O 2 is the change in p50 O 2 produced by a change in pH (ΔpH). The normal value of the Bohr effect at physiological pH and temperature is about 0.45.
What is the best method to measure oxygen dissociation?
The advantages and disadvantages of the various techniques have been reviewed. 59,60 The standard method with which new methods are compared is the gasometric method of Van Slyke and Neill. 61 This method is slow, demands considerable expertise and is not suitable for most haematology laboratories. Commercial instruments are now available for performing the test and drawing the complete oxygen dissociation curve, for example, Hemox Analyzer ( www.tcssci.com ). Such analysers are extremely quick and accurate and are therefore ideal for laboratories performing multiple determinations. Approximate measurement of oxygen saturation of haemoglobin can also be obtained at the bedside by noninvasive pulse oximetry.
What determines the saturation of Hb?
From Figure 1.12 it is apparent that the partial pressure of oxygen ( P O 2) plays a major role in determining Hb saturation ( S O 2 ), i.e., the percentage of haem groups that are bound to O 2. The P O 2 is the partial pressure of dissolved oxygen, and its arterial level is determined by the efficiency of diffusion from alveolus to plasma. i.e., movement of O 2 from an area of high to an area of low P O 2. Although 100% saturation may, on the face of it, indicate efficient O 2 transport, the Hb content needs to be borne in mind when interpreting this (see Fig. 1.12 for details).
What is the oxygen saturation curve?
Oxygen saturation curve Physiology A curve that describes the relationship between Hb O2saturation and tension; defined by a sigmoid curve which reflects the interaction of the 4 Hb molecules involved in O2uptake, transport and release; a 'right shift' of the curve indicates ↓ Hb affinity for O2, as occurs in ↓ pH–ie, acidosis, ↑ temperature, ↑ PCO2, while a 'left shift' indicates ↑ O2affinity with ↑ pH, ↓ temperature, ↓ 2,3 DPG and ↓ PCO2Right shifts Acidosis, hyperthermia, alveolar hypoventilation, anemia Left shifts Alkalosis, hypothermia, hyperventilation, carboxyhemoglobinemia, hypophosphatemia, ↑ fetal Hb. See 2,3 DPG.
What does the curve on the haemoglobin curve mean?
The curve is S-shaped and shows that HAEMOGLOBINhas a high affinity for oxygen. Blood can become saturated at relatively low oxygen tensions, but a small drop in oxygen tension brings about a big fall in the saturation of the blood. If tissues use up oxygen, then haemoglobin responds by giving it up.
Why is hemoglobin attracted to oxygen?
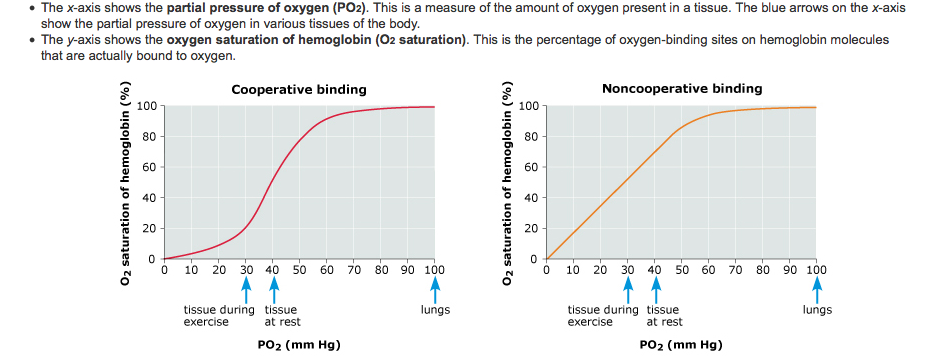
This is known as cooperative binding, and is the reason for the sigmoid shape of the oxygen dissociation curve. This also explains why hemoglobin is most attracted to oxygen when three of the four subunits are bound to oxygen.
Which group of water molecule is oxidized ferric iron bound to?
This oxidized ferric iron bounds to hydroxyl group of water molecule in which this complex has a shift toward the left in oxygen dissociation curveand is incapable to transport oxygen which leads to an ablated oxygenation in tissue with consequent hypoxic features.
Does CO displace oxygen?
CO can displace oxygen and lead to the shifting of the oxygen dissociation curveto the left.
Does dapsone cause methemoglobinemia?
Dapsone-induced methemoglobinemia in immune thrombocytopenia: A case report
Is oxygen saturation an insensitive reflection of arterial oxygen tension?
Owing to the sigmoid shape of the oxygen dissociation curvearterial oxygen saturation is an insensitive reflection of arterial oxygen tension.
What is the dissociation curve of oxygen and hemoglobin?
The oxygen-hemoglobin dissociation cur ve shows how the hemoglobin saturation with oxygen (SO2,), is related to the partial pressure of oxygen in the blood (PO2). Hemoglobin is the main protein within red blood cells, and it’s made of four globin subunits, each containing a heme group capable of binding one molecule of O2.
What is the main factor that influences oxygen saturation?
The main factor that influences oxygen saturation is the partial pressure of oxygen in the blood, measured in millimeters of mercury (mm Hg).
How saturated is hemoglobin?
And why in the venous capillaries of tissues, where the partial pressure of oxygen is about 40mmHg, hemoglobin is only about 75% saturated with oxygen .
What enzyme catalyzes the formation of carbonic acid in the blood?
Inside the red blood cell, the enzyme carbonic anhydrase catalyzes a reaction with CO2 and water which forms carbonic acid (H2CO3).
What is the state of oxygen binding to hemoglobin?
All of these states - where oxygen is bound to hemoglobin - are called oxyhemoglobin, changing to its relaxed state, or R-state with each O2 molecule that binds.
What shape does a curve take when plotted?
And when these points are plotted, the curve takes on a sigmoidal shape .
Why is O2 unloaded?
So, for example, when muscles are working hard, like during exercise, they are producing a lot more CO2, and, as a result, more H+ , which causes the pH to drop. So O2 is unloaded in the issues that need it most.