Molarity And Mole Fraction
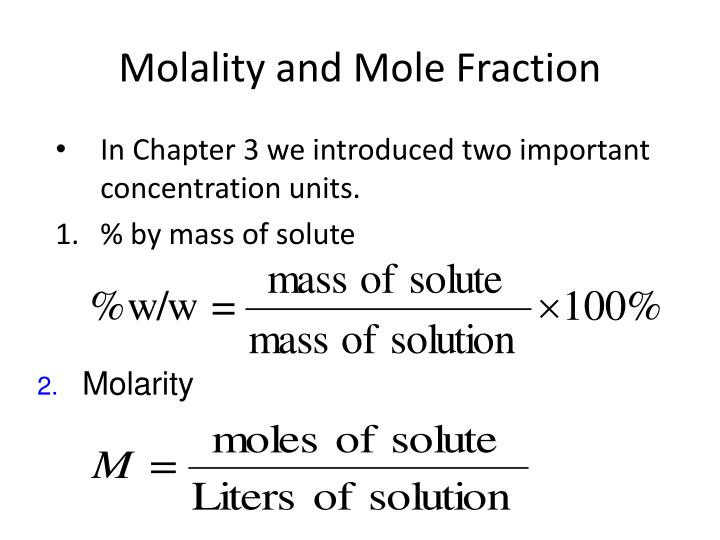
- Molarity. It is one of the most widely used unit of concentration and is denoted by M. ...
- Mass Per Cent or weight percent (w/w %) It is the ratio of the mass of solute to the mass of solution multiplied by 100 to calculate mass percent.
- Molality. It is defined as moles of solute present in 1-kilogram of solvent. ...
- Mole Fraction. ...
What is the relation between mole fraction and molality?
What is the formula (s)?
- Relationship b/w Molarity (M) or molality (m): m=1000×M/1000×D-M×Molar mass of solute Where D is density .
- Rel.b/w. molality (m) and mole fraction (X): m=1000×X of solute /X of solvent × molar mass of solvent
- Rel. ...
- M ,when density is given:-
What is the equation for calculating from moles to molarity?
- molar mass of K = 39.1 g
- molar mass of Mn = 54.9 g
- molar mass of O = 16.0 g
- total molar mass = K + Mn + O + O + O + O = 39.1 + 54.9 + 16 + 16 + 16 + 16 = 158 g
How do you calculate molality from molarity?
So follow these steps:
- Simply divide the Normality with the n-factor. Now we have the number of moles of solute per liter of solvent.
- Next divide 1000 by the weight of solvent. Thus you get the number of moles of solvent.
- Now we have total number of moles of solute and solvents.
- Now you can find the molality as per requirement of solute or solvent.
How to use molality to calculate mole fraction?
Mole Fraction using Molality Solution
- Convert Input (s) to Base Unit
- Evaluate Formula
- Convert Result to Output's Unit
What is mole molarity and molality?
Molarity is the ratio of the moles of a solute to the total liters of a solution. The solution includes both the solute and the solvent. Molality, on the other hand, is the ratio of the moles of a solute to the kilograms of a solvent.
What is meant by mole fraction?
Definition of mole fraction : the ratio of the number of moles of one component of a solution or other mixture to the total number of moles representing all of the components.
What is mole and molarity?
Mole is a measurement of the number of substances, whereas molarity is a measurement of the concentration. • Molarity gives an idea of the amount of substances present in a mixture. • Molarity is given as moles of a substance in one volume of a solvent.
What is molarity?
1:408:58What is Molarity (M) - Definition - Formula - Solved problems - YouTubeYouTubeStart of suggested clipEnd of suggested clipSo it can be written as m is equal to n by v here n is the number of moles of solute. Remember onceMoreSo it can be written as m is equal to n by v here n is the number of moles of solute. Remember once again it is not the number of moles of solvent. And v is volume of solution.
What is the unit of molality?
The SI unit of molality is moles per kilogram (mol/kg). For example, a solution whose molality is given as 6 mol/kg is stated as 6 molal or 6 m.
What is the SI unit of molarity?
In chemistry, the most commonly used unit for molarity is the number of moles per liter, having the unit symbol mol/L or mol⋅dm⁻³ in SI unit.
What is molality example?
Molality is defined as the number of moles of solute present in 1000 gm of the solvent. For example, 1 molal NaOH solution means a solution with 1 mole of NaOH in 1 Kg water. Mathematically, \[\text{molality(m)} = \frac{\text{number of moles of Solute(n)}}{\text{weight of the solvent in Kg}}\].
What is the unit of mole fraction?
Mole fraction is a unit of concentration, defined to be equal to the number of moles of a component divided by the total number of moles of a solution. Because it is a ratio, mole fraction is a unitless expression.
What is molarity and example?
Molarity is the number of moles of solute per liter of solution. For example, if you dissolve table salt in water, salt is the solute, and water is the solution. One mole of sodium chloride weighs 58.44 grams. If you dissolve 58.44 grams of NaCl in one liter of water, you have a one molar solution, abbreviated as 1M.
What is the mole formula?
Worked Example: moles = mass ÷ molar mass (n=m/M) Question: Calculate the amount of oxygen gas, O2, in moles present in 124.5 g of oxygen gas.
What is unit of normality?
What is Normality? Normality is a measure of concentration that is equal to the gram equivalent weight of solute per litre of solution. Gram equivalent weight is a measure of the reactive capacity of a molecule*. Unit of normality is Eq/L. “N” is the symbol used to denote normality.
What is mole for?
A mole is a very important unit of measurement that chemists use. A mole of something means you have 602,214,076,000,000,000,000,000 of that thing, like how having a dozen eggs means you have twelve eggs. Chemists have to measure using moles for very small things like atoms, molecules, or other particles.
What is mole fraction 11th chemistry?
Mole fraction is a unit of concentration. In the solution, the relative amount of solute and solvents are measured by the mole fraction and it is represented by “X.” The mole fraction is the number of moles of a specific component in the solution divided by the total number of moles in the given solution.
What is mole fraction class 12?
Mole fraction represents the number of molecules of a particular component in a mixture divided by the total number of moles in the given mixture.
How do you find the mole fraction?
Calculate the mole fraction of solute by dividing the moles of solute by the total number of moles of substances present in solution.
What is the mole fraction of h2o?
Mole fraction of A in H 2 O is 0.2.
What is molarity molality and mole fraction?
Molarity is the ratio of a solvent’s moles to a solution’s total litres. Both the solute and the solvent are part of the solution. Molality, on the...
How is mole fraction related to Molality?
The ratio of the number of moles of that component present in the solution to the total number of moles of all the solution components is known as...
How do I calculate moles?
Use the molecular formula to find the molar mass; divide the mass of the compound by the molar mass of the compound, represented in grammes, to obt...
How do you find molarity of HCl?
HCl moles are in a solution of 25.0 mL. Molarity is the ratio of solute moles and solution length. We can obtain the acid solution molarity by divi...
What is molarity example?
You split the moles of solute by the litres of solution to get the molarity. In a litre of solution, for example, a 0.25 mol/L NaOH solution contai...
What is the molarity of a solution?
Molarity (M), is the number of moles of solute (soluted substance) per unit volume of solution.
What is the molality of a substance?
Molality: It is defined as the moles of a substance/solute in 1 kg of a solvent. If 300 g of sugar (molar mass: 342 g/mol) is dissolved in 1500 g of water, the molality would be number of moles (342/300=0.87 moles) divided by 1500/1000 = 1.5 kg of solvent. Thus molality will be 0.87/1.5 = 0.58 moles/kg.
How many moles of NaCl are in 100g?
Since 58.44 g gives 1 mole of NaCl molecules, 100g gives 1.71 moles of NaCl. Thus, the molarity would be 1.71/4 = 0.43 moles/L.
How much salt is in 1 kg of water?
A solution of 58,5 g of salt (1 Mole of NaCl) in 1 kg of water has a Molality of 1.
What is the sum of all the molar fractions of a solution?
It is evident that the sum of all the molar fractions of a solution is equal to 1.
Is molarity useful for chemical reactions?
Molarity is very practical for doing chemical reactions in solutions by just measuring volumes.
Is there a formula for relating concentration terms?
There is no direct formula which relates the three concentration terms, but you can formulate one. But I think there is no such need.
What is the mole fraction of a component?
The mole fraction, X, of a component is the ratio of its molar amount to the total number of moles of all solution components: Molality is a concentration unit defined as the ratio of the numbers of moles of solute to the mass of the solvent in kilograms: Since these units are computed using only masses and molar amounts, ...
Why do molar concentrations vary?
Because solution volumes vary with temperature, molar concentrations will likewise vary. When expressed as molarity, the concentration of a solution with identical numbers of solute and solvent species will be different at different temperatures, due to the contraction/expansion of the solution.
Is mole fraction a dimensionless property?
Notice that mole fraction is a dimensionless property, being the ratio of properties with identical units (moles).
Do molar units vary with temperature?
Since these units are computed using only masses and molar amounts, they do not vary with temperature and, thus, are better suited for applications requiring temperature-independent concentrations, including several colligative properties, as will be described in this tutorial module.
What is the molarity of a solution?
Molarity of solution = Number of moles of the solute/volume of solution in L = 0.25/0.09346 =2.675 M
How to find the number of moles of a solute?
Number of moles of solute = Molarity of solution x volume of solution in L = 4.22 x 1 = 4.22
What is the molarity of NaOH?
Ans: The molarity of solution is 2.675mol L -1 or 2.675 M, the molality of solution is 2.778 mol kg -1 or 2.778 m, the mole fraction of NaOH is 0. 0476
What is the mole fraction of HNO3?
Ans: The mole fraction of HNO3 is 0. 0382, the molarity of solution is 2.011 mol L -1 or 2.011 M, the molality of solution is 2.206 mol kg -1 or 2.206 m
How much urea is dissolved in 100 g of water?
11.11 g of urea (NH2CONH2) was dissolved in 100 g of water. Calculate the molarity and molality of the solution. Given N = 14, H = 1, C = 12, O = 16.
What is the density of 5.35 M H2SO4?
The density of 5.35 M H2SO4 solution is 1.22 g cm-3. What is molality of a solution?
What is the density of NaOH in water?
An aqueous solution of NaOH is marked 10% (w/w). The density of the solution is 1.070 g cm-3. Calculate molarity, molality and mole fraction of NaOH in water. Given Na = 23, H =1 , O = 16