What is Cardiomyocyte necrosis and what causes it?
Cardiomyocyte necrosis: Alternative mechanisms, effective interventions. Abstract. Necrotic death of cardiac myocytes is a major contributor to heart failure associated with several cardiac pathologies such as ischemia and reperfusion injury.
What is necrosis and what causes it?
Necrosis is the death of the cells in your body tissues. Necrosis can occur due to injuries, infections or diseases. Lack of blood flow to your tissues and extreme environmental conditions can also cause necrosis. While dead body tissue can be removed, it can’t be brought back to good health.
What is the pathophysiology of myocardial necrosis?
During myocardial necrosis, myocytes loose membrane integrity and thus intracellular proteins diffuse into the interstitial space. The proteins ultimately enter into the cardiac microvasculature and lymphatics. Eventually, the proteins are detectable in the peripheral circulation and used as cardiac biomarkers of myocardial necrosis.
What happens to intracellular proteins during myocardial necrosis?
During myocardial necrosis, myocytes loose membrane integrity and thus intracellular proteins diffuse into the interstitial space. The proteins ultimately enter into the cardiac microvasculature and lymphatics.
What can cause myocardial necrosis?
Myocardial necrosis and mineralization can result from a number of causes, including nutritional deficiencies, chemical and plant toxins, ischemia, metabolic disorders, heritable diseases, and physical injuries (see Box 10-5).
What is myocardial cell necrosis?
Myocardial injury or myocardial necrosis refers to the cell death of cardiomyocytes and is defined by an elevation of cardiac troponin values. It is not only considered a prerequisite for the diagnosis of myocardial infarction but also an entity in itself and can arise from non-ischemic or non-cardiac conditions.
What is myocyte damage?
Myocyte damage and loss of myofibers is the potential mechanism of iron overload toxicity in congestive cardiac failure in thalassemia. Complete reversal of the cardiomyopathy and normalization of iron load by deferiprone. Hemoglobin.
Is myocardial necrosis fatal?
Abstract It is too often deduced that myocardial infarction is due to coronary occlusion and that subsequent death needs no other explanation. But the great majority of myocardial infarctions are not fatal, whether treated or untreated.
How are necrotic myocardial cells removed?
Ultimately the necrotic cells are removed by phagocytosis of the cellular debris by infiltrating leukocytes.
How does necrosis affect the heart?
Summary: The prevalence of heart failure continues to increase in the Western world, making it one of the biggest killers in this region. It is characterized by loss of the muscle cells of the heart (cardiomyocytes).
What is the definition of myocyte?
Medical Definition of myocyte : a contractile cell specifically : a muscle cell.
What do myocytes do?
The muscle myocyte is a cell that has differentiated for the specialized function of contraction. Although cardiac, skeletal, and smooth muscle cells share much common functionality, they do not all share identical features, anatomical structures, or mechanisms of contraction.
Is myocardial cell death reversible?
MI and HF are associated with significant loss of cardiac myocytes, a process that has been thought to be irreversible.
What are the 4 types of necrosis?
In addition to liquefactive and coagulative necrosis, the other morphological patterns associated with cell death by necrosis are:Caseous Necrosis.Fat Necrosis.Gangrenous Necrosis.Fibrinoid necrosis.
Where does myocardial necrosis occur?
Myocardial infarction (MI) is necrosis and death of heart muscle secondary to ischemia and acute coronary artery thrombosis. Thrombal occlusion of epicardial coronary arteries leads to cell death of the underlying subendocardium.
What is the life expectancy after myocardial infarction?
About 68.4 per cent males and 89.8 per cent females still living have already lived 10 to 14 years or longer after their first infarction attack; 27.3 per cent males, 15 to 19 years; and 4.3 per cent, 20 years or longer; of the females, one is alive 15 years, one 23 years and one 25 years or longer.
Where does myocardial necrosis occur?
Myocardial infarction (MI) is necrosis and death of heart muscle secondary to ischemia and acute coronary artery thrombosis. Thrombal occlusion of epicardial coronary arteries leads to cell death of the underlying subendocardium.
What are the 4 types of necrosis?
In addition to liquefactive and coagulative necrosis, the other morphological patterns associated with cell death by necrosis are:Caseous Necrosis.Fat Necrosis.Gangrenous Necrosis.Fibrinoid necrosis.
What type of necrosis is myocardial infarction?
The pathological hallmark of acute MI is coagulative necrosis of the myocardium.
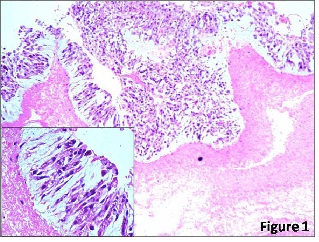
What type of necrosis is in the heart?
Coagulation necrosis (Figure 1) is the common pattern of necrosis characterized by cytoplasmic coagulation....Types of Myocardial Necrosis.NecrosisUnderlying MechanismCoagulation necrosisReduced vascular supplyColliquative myocytolysisLysis of cell structureCoagulation myocytolysisSympathetic nervous system hyper stimulationMay 9, 2016
Why is there no blood flow to the heart?
The main cause of reduced blood flow to the heart is a blockage or obstruction in the coronary arteries, which are the blood vessels that supply the heart with blood. Plaque and cholesterol can build up in the arteries, eventually blocking the blood flow, resulting in a heart attack and cellular death. This plaque and cholesterol build-up is caused by an unhealthy lifestyle.
What is myocardial necrosis?
Myocardial infarction necrosis is a condition of heart cell death caused during a heart attack. The cells of the heart require oxygen from the blood. If blood flow is decreased or blocked, the cells of the heart could die due to a lack of oxygen. The main cause of reduced blood flow to the heart is a blockage or obstruction in ...
Why is my heart unable to flow blood?
The main cause of reduced blood flow to the heart is an obstruction in the coronary arteries, the blood vessels that provide the heart with blood. These obstructions are often caused by the build-up of plaque and cholesterol due to years of unhealthy living, especially eating unhealthy foods and not exercising regularly.
What is bypass surgery?
Bypass surgery reroutes the coronary artery around the obstruction.
What is the cause of myocardial infarction?
Myocardial Infarction Necrosis: Causes. Myocardial infarction is another name for heart attack. The heart, like any other organ or muscle in the body, requires oxygen from blood in order to function and survive. During a heart attack, blood flow is reduced to the heart, resulting in the cells of the heart not receiving enough oxygen.
What causes a heart attack?
Heart attacks are often caused by blockages in the coronary arteries that impede blood flow to the heart. Foods that contribute to the development of plaque or cholesterol in the arteries include high-fat food foods such as fried foods and desserts, as well as foods and drinks high in sugar (such as sodas).
How old is Doug from Myocardial Infarction Necrosis?
Myocardial Infarction Necrosis. Doug is a 56-year-old college English Literature professor. He lives a pretty unhealthy lifestyle, eating fast food many times each week and rarely getting any physical activity. During one of his lectures, Doug began feeling a tightness and pressure in his chest and left shoulder, as well as shortness of breath.
What is the sequence of events involved in healing of the postinfarcted heart?
The sequence of structural events involved in healing of the postinfarcted heart has been well characterized in animals and humans. 28,29 However, the mechanisms by which the various cell populations present in the acute and subacute phases of the repair process are removed from the damaged myocardium are not clear. The study of Takemura et al 2 documents that cell death by apoptosis occurs in all cell types, providing important information on the remodeling of the ventricle after a 30-minute occlusion of the coronary artery and a reperfusion period of 2 days and 2 and 4 weeks. Although it is not surprising that inflammatory cells, consisting mostly of leukocytes, undergo apoptotic death, 30 it is somehow unexpected that myofibroblasts die in the same manner. Myofibroblasts are wound-healing cells with structural characteristics of smooth muscle cells and fibroblasts. 31 They are implicated in wound contraction 32 and, in the infarcted human heart, persist for several years and maintain an orientation parallel to the endocardium and epicardium. 29 This type of alignment, however, is detected only in transmural infarcts. Conversely, myofibroblasts are distributed in the direction of the longitudinal axis of myocytes in small patchy lesions of the wall. 29 Apoptosis occurs in endothelial cells, 2 and this phenomenon may be responsible for the progressive loss of vessels and reduction in blood supply to the healing region. Myofibroblasts counteract, at least in part, local ischemia, remaining a permanent component of the completed scar. The disappearance of the vascular framework with time may induce necrosis of interstitial cells and myofibroblasts as well. The recognition that apoptosis plays a role in tissue repair of the heart is significant, but whether cell necrosis participates in the reduction of proliferating cells and vascular structures in the healing myocardium is unknown. Moreover, the humoral and/or mechanical signals implicated in the activation of the endogenous cell death pathway in myofibroblasts, fibroblasts, macrophages, and endothelial cells remain to be identified. Myofibroblasts isolated from the infarcted myocardium synthesize and secrete angiotensin II, 33 and the scarred noncontracting myocardium is exposed to the physical forces resulting from systolic and diastolic pressure. These 2 events may contribute to the activation of apoptosis. Inflammatory cells and macrophages contain various cytokines and proteases, which, together, may be involved in the initiation of cellular disruption and death.
When was myocyte loss first discovered?
Myocyte loss was introduced in the late 1980s and early 1990s as a potential etiological factor of ventricular dysfunction in the aging heart of animals and humans. 20,21 However, the interest in myocyte loss exploded nearly 3 to 4 years ago when apoptosis was proposed as a form of cell death in the diseased heart. The number of studies on this subject has increased exponentially, and, currently, little attention is given to myocyte necrosis, 4,22,23 the type of cell death that was considered the only one occurring in the myocardium. Even in the old literature concerning prenatal development of the heart, myocyte death is discussed, 24 but apoptosis is not claimed as the mechanism. Currently, very little effort is made to establish whether myocytes die by apoptosis only, by necrosis exclusively, or by a combination. The analysis of cell death in vivo and in vitro is almost invariably restricted to apoptosis, and necrosis is essentially ignored. This is surprising and scientifically wrong. Myocyte necrosis is a major component of the decompensated heart and can affect large groups of cells, resulting in foci of replacement fibrosis, 25 or can occur in a scattered manner across the ventricular wall, 22 mimicking the distribution of apoptosis.
Can myosin monoclonal antibody be used in vitro?
The recognition that loss of myocytes involves apoptosis, necrosis, and apoptosis/necrosis requires the development of methodologies capable of detecting these types of cell death, particularly necrosis. In vitro preparations and in vivo animal studies do not represent a serious problem, because myosin monoclonal antibody can be added to the culture medium or administered in vivo. 4,22,23 In vitro, ethidium monoazide bromide (EMB) can be used to reveal small areas of damage in the sarcolemmal membrane, 26 or cells can be exposed to 5-hexadecanoylamino-fluorescein (HEDAF). 27 Additionally, propidium iodide (PI) labeling of nuclei can be used. 1 However, the analysis of myocyte necrosis in the human heart is complicated by the impossibility of injecting myosin antibody in vivo to label dying cells, and EMB, HEDAF, and PI cannot be used in tissue sections of the myocardium. Conversely, myocyte apoptosis can be detected histochemically and morphologically, but whether programmed cell death affects a myocyte independently from necrosis or whether necrosis is present in adjacent or distant myocytes cannot be established. This is a critical issue because apoptosis involves 0.2% of myocytes in end-stage failure, 17 a value that may challenge the significance of cell death in the final stage of the disease. On the other hand, myocyte necrosis may be comparable or may exceed apoptosis, and the combination of necrosis and apoptosis could decrease markedly the number of functioning cells in the heart. These comments are meant to emphasize the need to develop probes able to detect cell necrosis in situ. Of relevance, an accurate quantitative evaluation of the effects of apoptosis and necrosis on the diseased heart would require knowledge of the time necessary for the completion of each form of myocyte death. The rate of myocyte loss could then be calculated, and this information could be highly relevant in predicting the evolution of the overloaded decompensated heart.
Is acidosis a major etiological factor in myocyte apoptosis?
An interesting question raised by the observation that acidosis may be a major etiological factor in myocyte apoptosis is whether gene expression is required or implicated in the initiation and progression of the death process. p53 is upregulated in hypoxia, 14 and inhibition of VPATPase is characterized by the induction of p53 and p53-dependent genes such as p21. 7 However, the magnitude of apoptosis in the infarcted myocardium is not altered in mice nullizygous for p53. 15 Additionally, the expression of Bcl-2 is increased in ischemic myocytes, whereas Bax protein remains constant, 4 pointing to the lack of p53 activation under this setting. The Bcl-2 family of proteins constitutes a critical checkpoint in cell death. 16 These proteins contain agonists and antagonists of apoptosis, and alterations in their ratio determine the life or death of a cell. p53 is a transcriptional regulator of the bcl-2 and bax genes 16: p53 downregulates the antiapoptotic gene product Bcl-2 and upregulates the proapoptotic gene product Bax. Currently, it is a matter of controversy whether p53 and p53-inducible genes are involved in the modulation of myocyte apoptosis in ischemia.
What causes necrosis in the body?
Necrosis is caused by a lack of blood and oxygen to the tissue. It may be triggered by chemicals, cold, trauma, radiation or chronic conditions that impair blood flow. 1 There are many types of necrosis, as it can affect many areas of the body, including bone, skin, organs and other tissues. It isn't always a clot or cold ...
What is the type of necrosis that occurs when a clot forms in a blood vessel?
Another type of necrosis happens when a clot, such as a deep vein thrombosis (DVT) forms in a blood vessel and blocks blood flow to an area of the body.
Why do my lungs turn black after a frost bite?
One common type of necrosis is caused by damage from frostbite. During frostbite, the tissues are severely damaged by cold, and if the condition is not treated quickly, the frostbitten areas turn black and die. 2 These black areas are necrotic, or affected by necrosis, and cannot be healed and are typically removed during surgery.
What to do if you have a blockage in your blood?
Treatment may include surgery to restore blood flow or to remove the damaged tissues , antibiotics to prevent or treat infection, or treating the burn or other issues ...
Does Verywell Health use peer reviewed sources?
Verywell Health uses only high-quality sources, including peer-reviewed studies, to support the facts within our articles. Read our editorial process to learn more about how we fact-check and keep our content accurate, reliable, and trustworthy.
Can a car accident cause necrosis?
Any time blood flow is blocked to an area, or an area is so damaged that blood can not flow to and from it , necrosis may be possible.
Can a dead tissue be removed from the body?
Necrosis in the death of tissues of the body. Necrosis can be treated, with the dead tissue being removed, but the affected tissue can not be returned to good health. 1
What are the biochemical indicators of myocardial necrosis?
Biochemical indicators of myocardial necrosis are troponins T or I, the concentration considered as determining necrosis is that exceeding at least one value above the upper reference limit (99th percentile of the values for a reference control group) on at least one occasion during the first 24 h after the index clinical event. Myocardial injury with necrosis may also be detected with heart failure (HF), renal failure, myocarditis, arrhythmias, and pulmonary embolism and these are not changes due to STEMI damage or a complication of the procedures to resolve it. It is important to distinguish a rise and/or fall of cardiac troponin (cTn) values, from chronic elevations that tend not to be change acutely [1,2].
What are the baroreceptors in the heart?
Baroreceptors within the carotid sinus and aortic arch as well as mechanoreceptors within the myocardium provide an inhibitory signal for sympathetic output from the central nervous system in healthy individuals. A decrease in cardiac output or effective arterial volume will result in decreased output from these baroreceptors and increase signaling with sympathetic hormones, including norepinephrine and epinephrine ( Hartupee and Mann, 2016; Floras and Ponikowski, 2015 ). Early in the development of HF, these neurohormonal changes are seen due to decreased effective cardiac output. The sympathetic signaling increases as a compensatory mechanism to improve end-organ perfusion through inotropic and chronotropic effects.
What causes myocardial necrosis?
Myocardial necrosis and mineralization can result from a number of causes, including nutritional deficiencies, chemical and plant toxins, ischemia, metabolic disorders, heritable diseases, and physical injuries (see Box 10-5 ). From this large list of causes of myocardial injury, some of the most frequently observed current examples are ionophore ...
What biochemical markers are released during myocardial necrosis?
Myocardial necrosis is accompanied by the release of several biochemical markers in circulating blood, including creatine kinase, myoglobin, troponins T and I, and lactate dehydrogenase.
What are the effects of SNS activation?
In addition to the direct physical changes seen due to SNS activation, chronically heightened sympathetic tone has intracellular effects . As described previously sympathetic neuroendocrine hormones, norepinephrine and epinephrine, act through the G-protein-coupled beta-adrenergic receptors which increase inotropy, heart rate, and peripheral vascular resistance. This compensatory mechanism leads to increased myocardial stress through upregulation of cyclic adenosine monophosphate which produces elevated levels of intracellular calcium. Ultimately, the rise in cellular calcium and oxidative stress leads to cellular death. In addition, the cellular shifts in calcium appear to promote after depolarizations and ventricular arrhythmias ( Floras and Ponikowski, 2015; Sackner-Bernstein JaM, 1995; Ponikowski et al., 2001; Lown and Verrier, 1976 ).
What is the most common specimen encountered with hypersensitivity myocarditis?
Currently, the most common specimen encountered with hypersensitivity myocarditis is the explanted heart of the patient undergoing transplantation. In one reported series [ 22 ], hypersensitivity myocarditis was observed in cardiac tissue of 7.4% of those undergoing heart transplantation. Peripheral eosinophilia was present in 86% of patients.
Can interleukin-2 cause myocardial necrosis?
Some agents may cause myocardial necrosis without an inflammatory component, such as catecholamines and interleukin-2 [ 21 ]. More often, a toxic reaction is associated with myocarditis ( Figure 18.4 ). Examples of such agents also include catecholamines, and antimony, arsenicals, cyclophosphamide, emetine, lithium, and phenothiazines. While these agents are directly toxic to myocytes, others cause an immune response that involves the heart with or without other organ involvement. The entity is referred to as hypersensitivity myocarditis [ 22, 23 ].
Abstract
Necrotic death of cardiac myocytes is a major contributor to heart failure associated with several cardiac pathologies such as ischemia and reperfusion injury. Preventing cardiomyocyte necrosis is an important challenge towards the development of effective strategies, aiming to battle cardiovascular disorders.
Keywords
Three major types of cell death have been described in diverse organisms, apoptosis autophagic vacuolation and necrosis [1], [2], [3].
What is NAM in medical terms?
Definition. Necrotizing autoimmune myopathy (NAM) is a rare form of idiopathic inflammatory myopathy characterized clinically by acute or subacute proximal muscle weakness, and histopathologically by myocyte necrosis and regeneration without significant inflammation.
What is the diagnosis of muscle necrosis?
Diagnosis is based on the clinical picture and on muscle biopsy showing minimal or no inflammatory infiltrates and marked muscle necrosis, unlike other inflammatory myopathies. Electromyography (EMG) shows myopathic findings. Creatine kinase (CK) levels are often more than 10 times above the upper limit of normal at the time of onset of muscle weakness. Magnetic resonance imaging (MRI) may show diffuse or patchy edema within muscles. Anti-SRP and anti-HMGCoAR autoantibodies are frequently associated with this condition. Currently, seronegative NAM represents 20-30% of the cases.
What age does NAM occur?
Age of onset ranges from 30 to 70 years of age in reported cases. The main presenting feature of NAM is subacute severe symmetrical proximal myopathy, with a markedly elevated creatine kinase (CK) level. Its presentation is similar to that of polymyositis (see this term) with upper and lower limb weakness causing difficulty in moving from a sitting position, climbing stairs, or lifting objects The neck flexor, pharyngeal, and respiratorymuscles may also be involved. Other manifestations include fatigue, weight loss dysphagia and dyspnea. Interstitial lung disease (see this term) and cardiac involvement have also been reported. The course is often severe but may be self-limiting and recovery may occur within weeks to months of discontinuing the causative agent, if identified.
What is the CK level?
Creatine kinase (CK) levels are often more than 10 times above the upper limit of normal at the time of onset of muscle weakness. Magnetic resonance imaging (MRI) may show diffuse or patchy edema within muscles. Anti-SRP and anti-HMGCoAR autoantibodies are frequently associated with this condition.
What is the cause of a syphilis?
Etiology. The disease is thought to be related to an immune response possibly triggered by drug therapy (statins), connective tissue diseases, or cancer. The exact mechanism underling the disorder is not known but some autoantibodies appear to be a likely cause. Malignancy may be involved.
Why do we post questions on GARD?
Questions sent to GARD may be posted here if the information could be helpful to others. We remove all identifying information when posting a question to protect your privacy. If you do not want your question posted, please let us know.
What is the National Institute of Neurological Disorders and Stroke?
The National Institute of Neurological Disorders and Stroke (NINDS) (NINDS) collects and disseminates research information related to neurological disorders. Click on the link to view information on this topic.
What is the strongest argument for myocyte formation in the adult heart?
The strongest argument in favor of new myocyte formation in the adult heart is the increase in myocyte number from birth to young adulthood in both animals and humans. 27 These data, obtained by well-validated morphometric methods, 27 are internally consistent because, in addition to demonstrating an increase in myocyte number, they show that the increase in cardiac mass during normal growth cannot be accounted for solely by myocyte hypertrophy. Moreover, the increase in myocyte number in the adult represents an underestimation of the actual number of myocytes formed due to the concurrent cell death with maturation. 27 These data strongly suggest that myocyte renewal occurs throughout life in the myocardium and it is part and parcel of cardiac homeostasis. Interestingly, the renewal rate increases significantly under a variety of pathological conditions characterized mainly by an increase in cardiac wall stress. 20,27
What is the role of p53 in myocardial function?
In contrast to the mechanisms of myocyte death in the infarcted myocardium, p53 and p53-dependent and regulated genes play a critical role in the acute adaptation of the nonischemic portion of the heart. Myocyte stretch induced by diastolic dysfunction promotes the release of angiotensin II ( Figures 1G and 1H) and the activation of AT 1 receptors. 13,14 Receptor activation leads to phosphorylation of p38-MAP kinase that, in turn, phosphorylates the C-terminal of p53 at Ser390. 15 Because p53 DNA binding sites are present in the promoter of angiotensinogen and AT 1 receptor, p53 enhances the myocyte renin-angiotensin system (RAS) and the formation of angiotensin II. 14 In addition, p53 downregulates Bcl-2 and upregulates Bax. 14,15 A decreased Bcl-2-to-Bax protein ratio makes myocytes more susceptible to death signals transmitted by angiotensin II. 15
Why is myocyte replication controversial?
The controversy surrounding the issue of myocyte replication has been due, at least in part, to the need to reconcile two apparently contradictory bodies of evidence: one documenting the irreversible withdrawal of myocytes from the cell cycle and the other documenting the existence of myocyte mitosis and cytokinesis. Confusion in the interpretation of some of the data, lack of information about the origin and nature of replicating myocytes, together with the absence of an appropriate conceptual framework for myocyte replication have prolonged the debate.
How to determine the magnitude of myocyte replication?
The magnitude of myocyte replication that occurs in the overloaded heart in the absence of coronary artery disease can be accurately determined by the absolute increase in cell number in the ventricular myocardium. This fact, by itself, constitutes an incontrovertible demonstration of new myocyte formation in the adult heart. 27 By this approach, myocyte proliferation has been demonstrated in animal models and humans with cardiac failure. 33,35–38 Increases in cell number up to 60% or more have been identified, indicating that the mammalian heart possesses a significant growth reserve and a large number of new myocytes can be formed in a relatively short time. More recently, we have identified very high levels of myocyte regeneration in patients with aortic stenosis undergoing valve replacement (unpublished data, 2002). However, myocyte death by apoptosis and necrosis is invariably present in the overloaded heart. This phenomenon complicates the estimation of the real amount of newly formed cells by any methodological procedure. Myocyte hyperplasia is underestimated by cell death and cell death is underestimated by myocyte regeneration. 27
What is the role of myocytes in the overloaded heart?
The underlying hemodynamic condition plays a major role in the growth response of myocytes in the overloaded heart with no signs of ischemic injury. If cardiac dysfunction is not present and the ventricle is hemodynamically compensated, myocyte hypertrophy is the predominant form of cell growth, and myocyte proliferation is not significantly above that in control hearts. 39 Conversely, myocyte multiplication becomes the major growth adaptation of the failing heart. 33,37 These relationships have been observed in humans and animals exposed to pressure overload. 35–38 Little information is available in volume overload-induced cardiac hypertrophy, 27 but some results support the conclusions reached for pressure overload. 40
How does myocardial infarction affect myocytes?
The inability of old myocytes to hypertrophy and/or replicate becomes apparent when myocardial infarction is induced in young adult animals and the replicating myocytes are BrdU-labeled before euthanasia. At 7 days after infarction, DNA replication is restricted to small cells and decreases progressively with increasing cell size ( Figure 4 I). There is an almost perfect antithetic relationship between cell replication and p16 INK4a expression. p16 INK4a positive cells do not reenter the cell cycle, and cycling cells do not express p16 INK4a. Neither hypertrophy nor replication occurs in very large myocytes. Myocytes 35 000 μm 3 in volume and larger do not reenter the cell cycle and their growth response is limited to cellular hypertrophy The fraction of myocytes with extreme dimensions and impaired growth increases with age, progressively affecting the response of the old heart to pathological stimuli. 44,45 The age-dependent increase in myocyte death, coupled with reduction in the coronary vasculature, further deteriorates the functional adaptation of the senescent heart. 27 These phenomena may explain why coronary heart disease and its complications are major risk factors in the elderly, and myocardial infarction is associated with increased morbidity and mortality in this population.
How does aging affect the heart?
The process of aging offers an extraordinary example of the effects that the changing balance between cell death and cell growth has on the pathological restructuring of the heart. The accepted paradigm claims that the number of ventricular myocytes is established at birth and these cells contract and maintain cardiac function until death of the organism. However, it is now clear that myocytes undergo continuous turnover and dying cells are constantly replaced by newly formed myocytes. In the aging heart, a subpopulation of myocytes undergoes DNA replication and mitosis, another subpopulation undergoes hypertrophy, and yet another group experiences apoptosis and necrosis. 27 There is no doubt that myocyte death occurs throughout the lifespan of the organism independently from cardiac diseases. In the normal heart, the rate of cell death increases with age and after middle age it is not balanced by a concomitant increase in new myocyte formation. The excess cell death results in a net reduction in myocyte number. 20,27 This smaller number of viable myocytes hypertrophies to preserve myocardial mass resulting in an old heart of normal or slightly decreased size but with enlarged parenchymal cells. 41 Thus, myocyte death, hypertrophy, and new myocyte formation characterize the aging heart.