
Are unsaturated fatty acids good or bad?
Unsaturated fats help lower a person’s levels of LDL cholesterol, reduce inflammation, and build stronger cell membranes in the body. They may also help a person reduce the risk of rheumatoid...
Are polyunsaturated fatty acids bad for You?
Polyunsaturated fats — along with monounsaturated fats — are considered healthy fats, as they may reduce your risk of heart disease, especially when substituted for saturated fats ( 1, 2, 3, 4 ). The two major classes of polyunsaturated fats are omega-3 and omega-6 fatty acids.
What are some good sources of unsaturated fat?
Unsaturated Fat Examples: Monounsaturated
- avocadoes
- canola oil
- cashews
- olive oil
- peanut butter
- peanuts
- sesame oil
- sesame seeds
What are the sources of unsaturated fats?
Unsaturated fat: includes polyunsaturated fat and monounsaturated fat. Both types are predominantly found in plant products. Examples of polyunsaturated fat food sources include soybean, sunflower, fish and corn oils. Monounsaturated fat is found in high content in olive, peanut, and canola oils.
What is the product of oxidation of unsaturated fatty acids?
The products are acetyl-CoA and a fatty acyl-CoA that has been shortened by two carbon atoms. The reaction is catalyzed by thiolase. Because each shortened fatty acyl-CoA cycles back to the beginning of the pathway, β-oxidation is sometimes referred to as the fatty acid spiral.
What does fatty acid oxidation means?
Fatty acid β-oxidation is the process by which fatty acids are broken down to produce energy. Fatty acids primarily enter a cell via fatty acid protein transporters on the cell surface. Once inside, FACS adds a CoA group to the fatty acid. CPT1 then converts the long-chain acyl-CoA to long-chain acylcarnitine.
Where are unsaturated fatty acids oxidised?
mitochondrial matrixβ-Oxidation of Unsaturated Fatty Acids Just like the saturated fatty acids cross the mitochondrial membrane with the help of carnitine shuttle (For more details read Activation and Transportation of Fatty acids via Carnitine Shuttle), unsaturated fatty acids also reach the mitochondrial matrix as fatty acyl-CoA.
What is the oxidation of fatty acids called?
The process of fatty acid oxidation, called beta oxidation, is fairly simple.
What are the 3 stages of fatty acid oxidation?
1) Dehydrogenation catalyzed by acyl-CoA dehydrogenase, which removes two hydrogens between carbons 2 and 3. 2) Hydration catalyzed by enoyl-CoA hydratase, which adds water across the double bond. 3) Dehydrogenation catalyzed by 3-hydroxyacyl-CoA dehydrogenase, which generates NADH.
What causes fatty acid oxidation?
Fatty Acid Oxidation Disorders (FAODs) are a group of rare inherited conditions caused by enzymes that do not work properly. A number of enzymes are needed to break down fats in the body (a process called fatty acid oxidation). Problems with any of these enzymes can cause a fatty acid oxidation disorder.
Why unsaturated fat is easily oxidized?
Unsaturated fats are more susceptible to oxidation than are saturated fats, meaning the more polyunsaturated a fat is, the faster it will go rancid. This is due to the more unstable double bonds, which allow more oxygen to react at those points.
What is difference between saturated and unsaturated fatty acids?
The difference between saturated and unsaturated fat lies in the number of double bonds in the fatty acid chain. Saturated fats lack double bonds between the individual carbon atoms, while in unsaturated fats there is at least one double bond in the fatty acid chain.
What are the examples of unsaturated fatty acids?
Unsaturated fatsOlive, peanut, and canola oils.Avocados.Nuts such as almonds, hazelnuts, and pecans.Seeds such as pumpkin and sesame seeds.
What does the oxidation mean?
(OK-sih-DAY-shun) A chemical reaction that takes place when a substance comes into contact with oxygen or another oxidizing substance. Examples of oxidation are rust and the brown color on a cut apple.
How are saturated fatty acids oxidized?
Mitochondrial β-oxidation of saturated straight-chain fatty acids is a catabolic pathway that involves the participation of a number of enzymes and generates acetyl-coA, NADH+ and FADH2 from fatty acyl-coA esters.
What are the products of fatty acid oxidation?
The end products of fatty acid oxidation include acetyl-CoA, FADH2, NADH, water and one acyl-CoA chain that is two carbons shorter. The acetyl-CoA enters the Krebs or citric acid cycle with FADH2 and NADH to yield ATP (adenosine triphosphate), which is used for energy.
What does the oxidation mean?
(OK-sih-DAY-shun) A chemical reaction that takes place when a substance comes into contact with oxygen or another oxidizing substance. Examples of oxidation are rust and the brown color on a cut apple.
Where does fatty acid oxidation occur?
Oxidation of fatty acids occurs in multiple regions of the cell within the human body; the mitochondria, in which only Beta-oxidation occurs; the peroxisome, where alpha- and beta-oxidation occur; and omega-oxidation, which occurs in the endoplasmic reticulum.
What are the products of fatty acid oxidation?
The end products of fatty acid oxidation include acetyl-CoA, FADH2, NADH, water and one acyl-CoA chain that is two carbons shorter. The acetyl-CoA enters the Krebs or citric acid cycle with FADH2 and NADH to yield ATP (adenosine triphosphate), which is used for energy.
Why is fatty acid oxidation called beta-oxidation?
It is named as such because the beta carbon of the fatty acid undergoes oxidation to a carbonyl group. Beta-oxidation is primarily facilitated by the mitochondrial trifunctional protein, an enzyme complex associated with the inner mitochondrial membrane, although very long chain fatty acids are oxidized in peroxisomes.
Oxidation of Monounsaturated (MUFA)
Unsaturated fatty acids such as Oleic acid are oxidized by same general pathway as saturated fatty acids.
Oxidation of Polyunsaturated Fatty acids (PUFA)
Polyunsaturated fatty acids like Linoleic acid, require a second auxiliary enzyme to complete the oxidation as they have 2 or more “cis” double bonds.
What are unsaturated fatty acids?
Unsaturated fatty acids are a component of the phospholipids in cell membranes and help maintain membrane fluidity. Phospholipids contain a variety of unsaturated fatty acids, but not all of these can be synthesized in the body.
How are unsaturated fatty acids converted to hydroxy fatty acids?
Unsaturated fatty acids (e.g. oleic acid) can be converted into hydroxy fatty acids by oxygenases or fatty acid double bond hydratases (Kim and Oh,2013 ). Thereby, unsaturated fatty acids can be subjected to the oxidative cleavage of C–C bond after hydroxylation or hydration of a double bond ( Jeon etal., 2016; Koppireddi etal.,2016; Song etal.,2013) ( Scheme3 ). One of the representative example was conversion of oleic acid into 9- (nonanoyloxy)nonanoic acid ( 15) via 10-hydroxyoctadecanoic acid and 10-keto-octadecanoic acid by a fatty acid double bond hydratase from Stenotrophomonas maltophilia, the SADH from M. luteus, and the BVMO from P. putida KT2440. As a result, n -nonanoic acid ( 16) and 9-hydroxynonanoic acid ( 17) could be produced to a high yield from oleic acid by a lipase from Thermomyces lanuginosa (TLL) and the recombinant E. coli strain expressing the fatty acid double bond hydratase from S. maltophilia, the SADH from M. luteus, and the BVMO from P. putida KT2440 ( Jeon etal.,2016 ).
How are alkoxyl radicals formed?
As mentioned earlier alkoxyl radicals are readily formed from decomposition of lipid hydroperoxides at high temperatures and/or in the presence of traces of transition metals. Alkoxyl radicals may further decompose to form a variety of non-volatile and volatile secondary oxidation products ( Frankel, 1991 ). The latter compounds are responsible for the flavor deterioration that is a result of lipid oxidation ( Aidos et al., 2002; Aro et al., 2003; Frankel, 1983; Kulås et al., 2002; Venkateshwarlu et al., 2004 ).
What is the reaction of peroxyl radicals?
The peroxyl radical reacts with a new unsaturated fatty acid to form hydroperoxides (LOOH) and a new lipid radical, which will subsequently propagate the chain reactions. Lipid hydroperoxides are the primary products of autoxidation. Because of their low volatility they are taste- and odorless.
How are unsaturated fatty acids biosynthesized?
Unsaturated fatty acids are typically biosynthesized by a unique dehydrogenation reaction called desaturation. The chemistry of this transformation is remarkable in its selectivity. The invention and application of novel mechanistic probes (deutero-, fluoro-, thia-containing substrate analogues) has led to a more sophisticated understanding of how fatty acid desaturases and related enzymes carry out their ultra-selective oxidation chemistry. The stereochemistry and order of hydrogen removal has been examined for a number of soluble and membrane-bound desaturses. Mechanistic models have been constructed to account for the results obtained. This knowledge can now be used to advance the design of medically relevant desaturase inhibitors and to guide protein engineering experiments in the area of plant biotechnology.
What enzyme produces unsaturated fatty acids?
Unsaturated fatty acids (UFA) are produced by the action of the enzyme, Δ-9- fatty acid desaturase, which inserts a double bond into fatty acid under aerobic conditions.
What are the odd numbered double bonds in fatty acids?
All double bonds present in unsaturated and polyunsaturated fatty acids can be classified either as odd-numbered double bonds, like the 9 -cis double bond present in oleic acid and linoleic acid, or as even-numbered double bonds like the 12- cis double bond of linoleic acid.
How are unsaturated fatty acids biosynthesized?
Unsaturated fatty acids are typically biosynthesized by a unique dehydrogenation reaction called desaturation. The chemistry of this transformation is remarkable in its selectivity. The invention and application of novel mechanistic probes (deutero-, fluoro-, thia-containing substrate analogues) has led to a more sophisticated understanding of how fatty acid desaturases and related enzymes carry out their ultra-selective oxidation chemistry. The stereochemistry and order of hydrogen removal has been examined for a number of soluble and membrane-bound desaturses. Mechanistic models have been constructed to account for the results obtained. This knowledge can now be used to advance the design of medically relevant desaturase inhibitors and to guide protein engineering experiments in the area of plant biotechnology.
What is the role of unsaturated fatty acids in the cell membrane?
Desaturation of Fatty Acids. Unsaturated fatty acids are a component of the phospholipids in cell membranes and help maintain membrane fluidity. Phospholipids contain a variety of unsaturated fatty acids, but not all of these can be synthesized in the body. •.
What are the double bonds in unsaturated fatty acids?
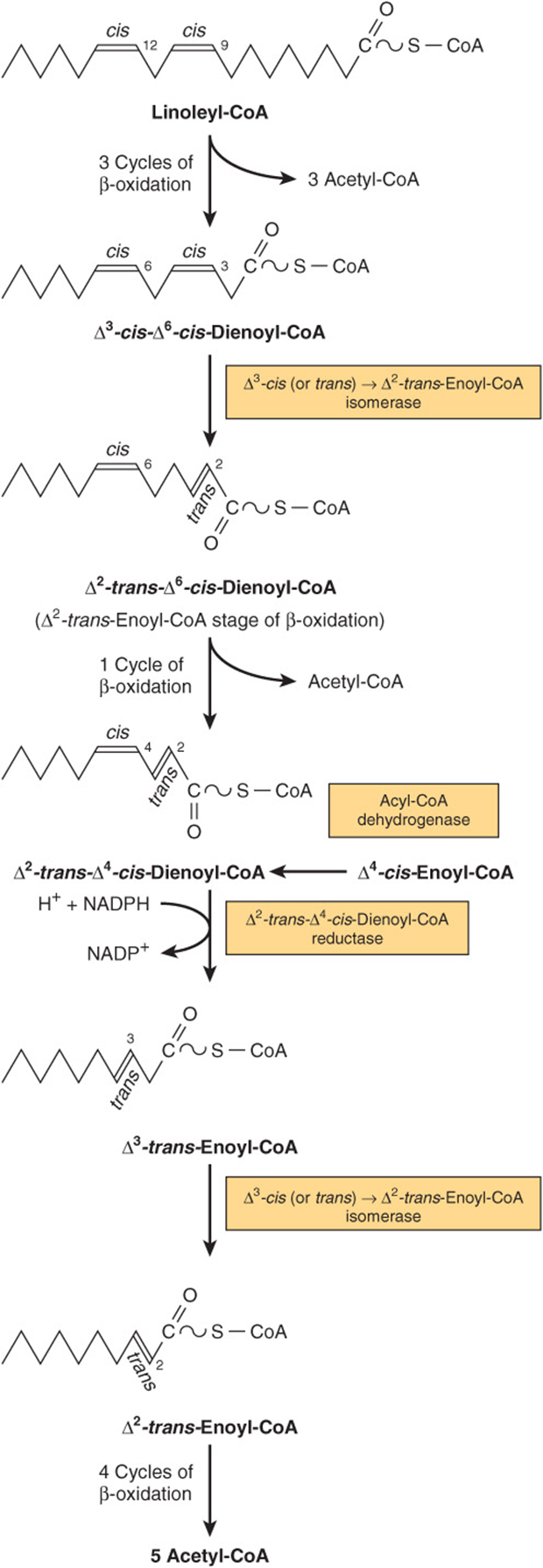
Unsaturated fatty acids, which usually contain cis double bonds , also are degraded by β -oxidation. However, additional (auxiliary) enzymes are required to act on the preexisting double bonds once they are close to the thioester group as a result of chain-shortening [17]. All double bonds present in unsaturated and polyunsaturated fatty acids can be classified either as odd-numbered double bonds, like the 9 -cis double bond present in oleic acid and linoleic acid, or as even-numbered double bonds like the 12- cis double bond of linoleic acid. Since both classes of double bonds are present in linoleic acid, its degradation illustrates the breakdown of all unsaturated fatty acids. A summary of the β -oxidation of linoleic acid is presented in Fig. 3. Linoleic acid, after conversion to its CoA thioester (I), undergoes three cycles of β -oxidation to yield 3- cis ,6- cis -dodecadienoyl-CoA (II) which is isomerized to 2- trans ,6- cis -dodecadienoyl-CoA (III) by ∆ 3, ∆ 2-trans -enoyl-CoA isomerase, an auxiliary enzyme of β -oxidation. 2- trans ,6- cis -Dodecadienoyl-CoA (III) is a substrate of β -oxidation and can complete one cycle to yield 4 -cis -decenoyl-CoA (IV) which is dehydrogenated to 2- trans ,4- cis -decadienoyl-CoA (V) by medium-chain acyl-CoA dehydrogenase. Long-chain acyl-CoA dehydrogenase does not act on 4- cis -decenoyl-CoA even though it is highly active with decanoyl-CoA. 2- trans ,4- cis -Decadienoyl-CoA (V) cannot continue on its course through the β -oxidation spiral, but instead is reduced by NADPH in a reaction catalyzed by 2,4-dienoyl-CoA reductase. The product of this reduction, 3- trans -decenoyl-CoA (VI), is isomerized by Δ 3 ,Δ 2 -enoyl-CoA isomerase to 2- trans -decenoyl-CoA (VII), which can be completely degraded by completing four cycles of β -oxidation. Therefore, the degradation of unsaturated fatty acids in mitochondria requires at least Δ 3 ,Δ 2 -enoyl-CoA isomerase and 2,4-dienoyl-CoA reductase as auxiliary enzymes in addition to the enzymes of the β -oxidation spiral.
What are the odd numbered double bonds in fatty acids?
All double bonds present in unsaturated and polyunsaturated fatty acids can be classified either as odd-numbered double bonds, like the 9 -cis double bond present in oleic acid and linoleic acid, or as even-numbered double bonds like the 12-cis double bond of linoleic acid.
What are the functions of fatty acids in the membrane?
Unsaturated fatty acids are responsible for the “fluid” nature of functioning biological membranes. It has been shown that it is the introduction of the first double bond to an acyl chain that is primarily responsible for its fluidity at normal physiological temperatures, and introduction of more and more double bonds to the acyl chain has relatively little additional effect on membrane fluidity. Thus substitution of fatty acids with four or six double bonds with those having only two (or sometimes three) double bonds will strongly decrease the susceptibility to lipid peroxidation while maintaining membrane fluidity. This phenomenon may be helpful in longevous animals, and in view of membrane acclimation to low temperature in ectotherms, has been called homeoviscous longevity adaptation [11].
What are the targets of oxidative modification?
Unsaturated lipids in cell membranes, including phospholipids and cholesterol, are well-known targets of oxidative modification, which can be induced by a variety of stresses, including ultraviolet A (UVA)- and visible light-induced photodynamic stress. Photodynamic lipid peroxidation has been associated with pathological conditions such as skin phototoxicity and carcinogenesis, as well as therapeutic treatments such as antitumor photodynamic therapy (PDT). Lipid hydroperoxides (LOOHs), including cholesterol hydroperoxides (ChOOHs), are important non-radical intermediates of the peroxidative process which can (i) serve as in situ reporters of type I vs. type II chemistry, (ii) undergo one-electron or two-electron reductive turnover which determines whether peroxidative injury is respectively intensified or suppressed, and (iii) mediate signaling cascades which either fortify antioxidant defenses of cells or evoke apoptotic death if oxidative pressure is too great. The purpose of this chapter is to review current understanding of photodynamic (UVA or visible light-induced) lipid peroxidation with a special focus on these aspects relating to LOOHs. Future goals in this area, many of which depend on continued development of state-of-the-art analytical techniques, will also be discussed.
What is LA metabolized to?
LA is metabolized to arachidonic acid (AA; 20 : 4n-6) and LNA to eicosapentaenoic acid (EPA; 20 : 5n-3) and docosahexaenoic acid (DHA; 22 : 6n-3 ), increasing the chain length and degree of unsaturation by adding extra double bonds to the carboxyl end of the fatty acid molecule ( Figure 2 ). Table 1.
What is the chemistry of oxylipins?
The chemistry, biochemistry, pharmacology and molecular biology of oxylipins (defined as a family of oxygenated natural products that are formed from unsaturated fatty acids by pathways involving at least one step of dioxygen-dependent oxidation) are complex and occasionally contradictory subjects t ….
What are oxylipins made of?
protectins, resolvins and maresins, or specialized pro-resolving mediators), eicosanoids and octadecanoids and plant oxylipins, which are deri ved from either the omega-6 (n-6) or the omega-3 (n-3) families of polyunsaturated fatty acids. For example, the term eicosanoid is used to embrace those biologically active lipid mediators that are derived from C20 fatty acids, and include prostaglandins, thromboxanes, leukotrienes, hydroxyeicosatetraenoic acids and related oxygenated derivatives. The key enzymes for the production of prostanoids are prostaglandin endoperoxide H synthases (cyclo-oxygenases), while lipoxygenases and oxidases of the cytochrome P450 family produce numerous other metabolites. In plants, the lipoxygenase pathway from C18 polyunsaturated fatty acids yields a variety of important products, especially the jasmonates, which have some comparable structural features and functions. Related oxylipins are produced by non-enzymic means (isoprostanes), while fatty acid esters of hydroxy fatty acids (FAHFA) are now being considered together with the oxylipins from a functional perspective. In all kingdoms of life, oxylipins usually act as lipid mediators through specific receptors, have short half-lives and have functions in innumerable biological contexts.
What is the oxidation of fats?
Rancidity is the oxidation of fats that is caused by hydration (water), oxidation (oxygen), metallic atoms or microbes. Rancidity often produces unusual odor and/or taste. Some unsaturated fatty acid fragments created by oxidation give food unique volatile qualities. Consider the aroma of crushed greens, cucumbers and deep-fried foods.
What are the functions of unsaturated fatty acids?
Unsaturated fatty acids are responsible for the “fluid” nature of functioning biological membranes. It has been shown that it is the introduction of the first double bond to an acyl chain that is primarily responsible for its fluidity at normal physiological temperatures, and introduction of more and more double bonds to the acyl chain has relatively little additional effect on membrane fluidity. Thus substitution of fatty acids with four or six double bonds with those having only two (or sometimes three) double bonds will strongly decrease the susceptibility to lipid peroxidation while maintaining membrane fluidity. This phenomenon may be helpful in longevous animals, and in view of membrane acclimation to low temperature in ectotherms, has been called homeoviscous longevity adaptation [11].
What are the targets of oxidative modification?
Unsaturated lipids in cell membranes, including phospholipids and cholesterol, are well-known targets of oxidative modification, which can be induced by a variety of stresses, including ultraviolet A (UVA)- and visible light-induced photodynamic stress. Photodynamic lipid peroxidation has been associated with pathological conditions such as skin phototoxicity and carcinogenesis, as well as therapeutic treatments such as antitumor photodynamic therapy (PDT). Lipid hydroperoxides (LOOHs), including cholesterol hydroperoxides (ChOOHs), are important non-radical intermediates of the peroxidative process which can (i) serve as in situ reporters of type I vs. type II chemistry, (ii) undergo one-electron or two-electron reductive turnover which determines whether peroxidative injury is respectively intensified or suppressed, and (iii) mediate signaling cascades which either fortify antioxidant defenses of cells or evoke apoptotic death if oxidative pressure is too great. The purpose of this chapter is to review current understanding of photodynamic (UVA or visible light-induced) lipid peroxidation with a special focus on these aspects relating to LOOHs. Future goals in this area, many of which depend on continued development of state-of-the-art analytical techniques, will also be discussed.
Why do unsaturated fatty acids kink?
The kinking creates a more fluid membrane because the molecules cannot be packed close together, unlike the saturated fatty acids, which can lie in a line. The kinking also ensures that cis unsaturated fatty acids are fluid at room temperature (which is better for areas such as artery walls). Trans fatty acids are problematic because the lipids can lie flat, like a saturated fatty acid, and so cause solid areas of rigid plaque in places like the arteries.
What are the odd numbered double bonds in fatty acids?
All double bonds present in unsaturated and polyunsaturated fatty acids can be classified either as odd-numbered double bonds, like the 9 -cis double bond present in oleic acid and linoleic acid, or as even-numbered double bonds like the 12-cis double bond of linoleic acid.
What are the functions of fatty acids in the membrane?
Unsaturated fatty acids are responsible for the “fluid” nature of functioning biological membranes. It has been shown that it is the introduction of the first double bond to an acyl chain that is primarily responsible for its fluidity at normal physiological temperatures, and introduction of more and more double bonds to the acyl chain has relatively little additional effect on membrane fluidity. Thus substitution of fatty acids with four or six double bonds with those having only two (or sometimes three) double bonds will strongly decrease the susceptibility to lipid peroxidation while maintaining membrane fluidity. This phenomenon may be helpful in longevous animals, and in view of membrane acclimation to low temperature in ectotherms, has been called homeoviscous longevity adaptation [11].
What is the role of arachidonate in the synthesis of lipids?
Arachidonate is an important component of membrane lipids and, together with linoleic and linolenic acid, serves as a precursor for the synthesis of prostag landins, thromboxanes, leukotrienes, and lipoxins. Fatty acid chains are polymerized in the cytoplasm and oxidized in the mitochondrial matrix.
Why do unsaturated fatty acids have double bonds?
Because double bonds can disturb the stereochemistryneeded for oxidative ...
What is the first step in the fatty acid oxidation process?
First, the fatty acid is activated. This involves the addition of a coenzyme A (CoA)molecule to the end of a long-chain fatty acid, after which the activated fatty acyl-CoA enters the β-oxidation pathway. Second, an oxidation step occurs.
How do fatty acids generate energy?
To generate energy from fatty acids, they must be oxidized. Fatty acid oxidation is also referred to as β-oxidation because two carbon units are cleaved off at the β-carbon position (second carbon from the acid end) of an activated fatty acid. β-oxidation that takes place in the matrix of the mitochondria and converts their fatty acid chains into two carbon units of acetyl groups, while producing NADH and FADH2. The acetyl groups are picked up by CoA to form acetyl-CoA that proceeds into the citric acid cycle as it combines with oxaloacetate. The NADH and FADH2 are then used by the electron transport chain.
What enzymes are used to degrade unsaturated fatty acids?
β-oxidation is the catabolic breakdown of fatty acids to produce energy; this process can completely degrade saturated fatty acids but requires the input of the enzymes enoyl-CoA isomerase and 2,4-dienoyl CoA, to complete degradation of unsaturated fatty acids.
What is the coenzyme A in fatty acids?
Stereochemistry: In chemistry, the spatial arrangement of atoms in a molecule. Coenzyme A: A coenzyme (protein) that is necessary for fatty acid synthesis and oxidation.
What are fatty acids?
Fatty acids: Lipids that contain a carboxylic acid functional group attached to a long-chain hydrocarbon tail. Satura ted fa tty acids: Fatty acids with hydrocarbon chains containing only single bonds (C-C). Unsaturated fatty acids: Fatty acids with hydrocarbon chains containing at least one double bond (C=C).
What is the term for the loss of electrons from a molecule to oxygen?
Oxidation: The loss of electrons from a molecule to oxygen; in the context of lipid metabolism, electrons are transferred from a fatty acid to oxygen, oxidizing the fatty acid. β-carbon: In a fatty acid, the second carbon from the carboxylic acid end.
What is the chemistry of oxylipins?from pubmed.ncbi.nlm.nih.gov
The chemistry, biochemistry, pharmacology and molecular biology of oxylipins (defined as a family of oxygenated natural products that are formed from unsaturated fatty acids by pathways involving at least one step of dioxygen-dependent oxidation) are complex and occasionally contradictory subjects t ….
What are oxylipins made of?from pubmed.ncbi.nlm.nih.gov
protectins, resolvins and maresins, or specialized pro-resolving mediators), eicosanoids and octadecanoids and plant oxylipins, which are deri ved from either the omega-6 (n-6) or the omega-3 (n-3) families of polyunsaturated fatty acids. For example, the term eicosanoid is used to embrace those biologically active lipid mediators that are derived from C20 fatty acids, and include prostaglandins, thromboxanes, leukotrienes, hydroxyeicosatetraenoic acids and related oxygenated derivatives. The key enzymes for the production of prostanoids are prostaglandin endoperoxide H synthases (cyclo-oxygenases), while lipoxygenases and oxidases of the cytochrome P450 family produce numerous other metabolites. In plants, the lipoxygenase pathway from C18 polyunsaturated fatty acids yields a variety of important products, especially the jasmonates, which have some comparable structural features and functions. Related oxylipins are produced by non-enzymic means (isoprostanes), while fatty acid esters of hydroxy fatty acids (FAHFA) are now being considered together with the oxylipins from a functional perspective. In all kingdoms of life, oxylipins usually act as lipid mediators through specific receptors, have short half-lives and have functions in innumerable biological contexts.
