
Peroxidase
A haem enzyme that catalyses reduction of hydrogen peroxide by a substrate that loses two hydrogen atoms. Within cells, may be localised in peroxisomes. Coloured reaction products allow detection of the enzyme with high sensitivity, so peroxidase coupled antibodies are widely used i…
Peroxidase
A haem enzyme that catalyses reduction of hydrogen peroxide by a substrate that loses two hydrogen atoms. Within cells, may be localised in peroxisomes. Coloured reaction products allow detection of the enzyme with high sensitivity, so peroxidase coupled antibodies are widely used i…
Why does an enzyme active site mutant show activity?
The active site of an enzyme is the region, which shows the highest metabolic activity by catalysing the enzyme-substrate complex into the products. The active site is found deep inside the enzyme, which resembles a hole or small depression.
Why does enzyme activity increase?
This is due to the increase in velocity and kinetic energy that follows temperature increases. This results in more molecules reaching the activation energy, which increases the rate of the reactions. Since the molecules are also moving faster, collisions between enzymes and substrates also increase.
How does enzyme actually catalyze any reaction?
How Do Enzymes Catalyze Chemical Reactions? Enzymes catalyze chemical reactions by first binding to molecules and then lining them up in ways that increase the probability of the molecules exchanging atoms when they collide. Enzymes therefore allow scientists to control the exchange of atoms mechanically, as explained by Science Daily.
How does enzyme activity vary with concentration?
The effect of salt concentration on the activity of enzymes may vary from one enzyme to another as some enzymes need salt in small amounts for their activities. In this case, if the concentration is very low or even is at zero, the amino acid chains forming the enzymes will attract each other, become precipitated, and denatured.
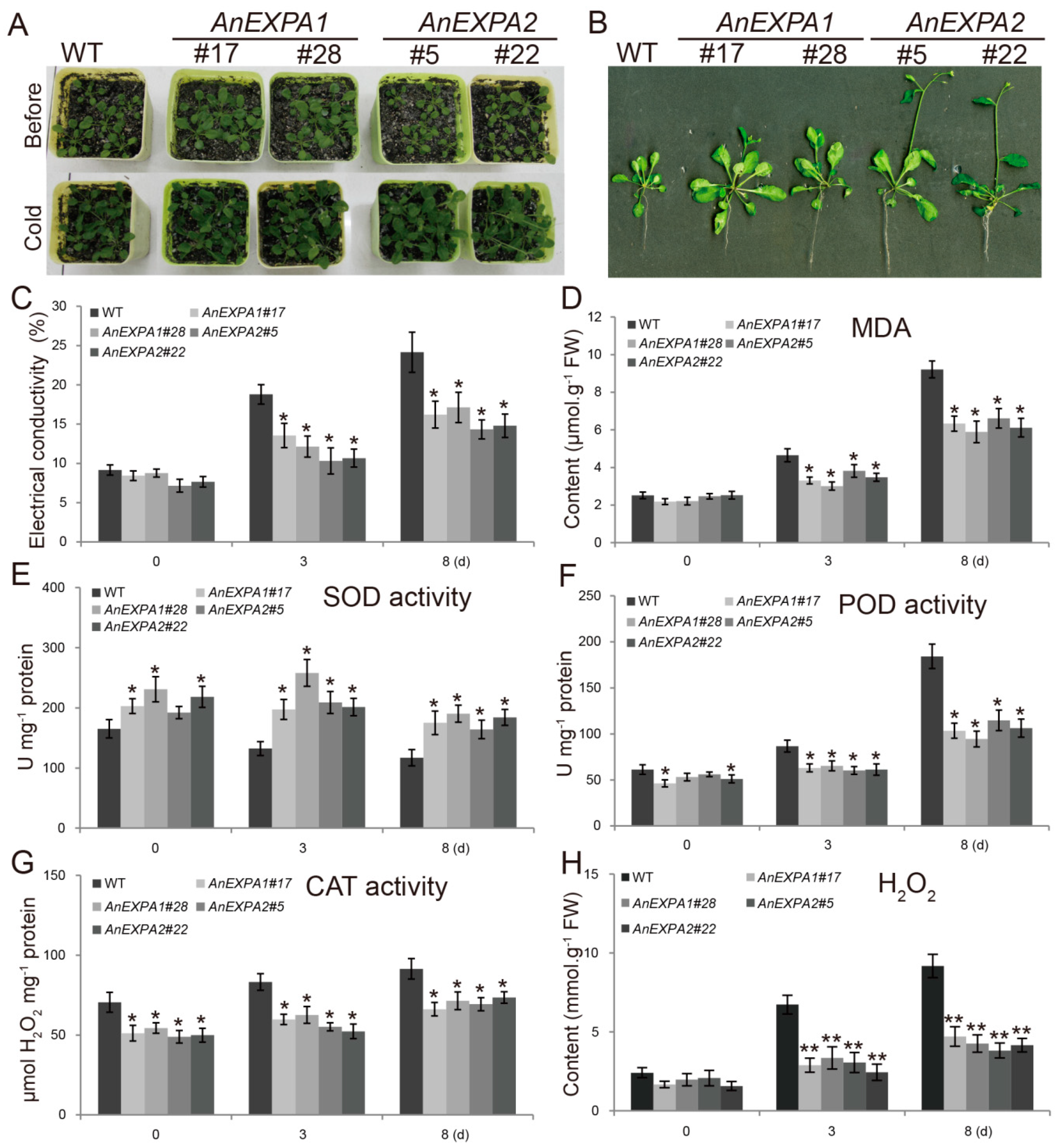
What is meant by peroxidase activity?
: an enzyme that catalyzes the oxidation of various substances by peroxides.
What is peroxidase activity of hemoglobin?
As a hemoprotein, hemoglobin (Hb) can, in the presence of H2O2, act as a peroxidase. In red blood cells, this activity is regulated by the reducing environment.
How is peroxidase activity determined?
2:213:44How to assay the Peroxidase activity? - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe principle is min pyro killall is added to hydrogen peroxide purple color purple gallon is formedMoreThe principle is min pyro killall is added to hydrogen peroxide purple color purple gallon is formed amount of pepper again informed determines the peroxidase activity.
What affects peroxidase activity?
The results of this experiment demonstrated that the performance of hydrogen peroxidase was greatly affected by all three factors, temperature, concentration level, and pH.
What is the importance of peroxidase in blood?
Peroxidase: A Clinically Important Enzyme These enzymes utilize hydrogen peroxide to catalyze the oxidation of variety of organic and inorganic compounds. Over the years the development in clinical and diagnostic techniques, redox natured reactions are gaining vital importance.
Where is peroxidase found in the human body?
Thyroid peroxidase also called as thyroperoxidase (TPO) is mainly expressed in thyroid organs. It is a large transmembrane glycoprotein with covalently linked haem, present in cells on the apical membrane (Gardas et al., 1999).
What type of enzyme is peroxidase?
Peroxidases are a group of enzymes that catalyze the oxidation of a substrate by hydrogen peroxide or an organic peroxide. Most peroxidases are ferric heme proteins – one notable exception being the glutathione peroxidase, which is a selenium-containing enzyme. They are present in virtually all living species.
What sorts of edible plants have peroxidase activity?
The peroxidase enzyme activities of some fresh vegetables (cabbage, leeks, carrot, spinach, celery, squash, potatoes, onions and green beans) were determined. The peroxidase activities of cabbage and green beans were high.
Is catalase a peroxidase?
Catalase and peroxidase are enzymes. The key difference between catalase and peroxidase is that catalase catalyzes the decomposition of hydrogen peroxide into water and oxygen, whereas peroxidase catalyzes the decomposition of peroxides. Therefore, catalase is a type of peroxidase enzyme.
How does pH affect peroxidase activity?
Peroxidase activity ceases at a pH of 2.5 or 8.5 to 9.5. Lower pH levels result in a more effective inactivation of the enzyme than do higher pH levels, meaning that acidic environments are more effective inhibitors of peroxidase activity than basic environments.
How does temperature affect peroxidase activity?
Activity of peroxidase even in 70 ºC was observed and is 10 % related to control (25 ºC). These results showed that, peroxidase show activity at high temperatures. A ten degree Centigrade rise from 40 to 50 ºC in temperature after 5 minute incubation will increase the activity of peroxidase from 170 to 70 %.
What was the optimal pH for peroxidase activity?
The optimal pH of the purified peroxidase was 5.0 and its activity was retained at pH values between 5.0–10.0. The enzyme was heat stable over a wide range of temperatures (0–60°C), and less than 50% of its activity was lost at 70°C after incubation for 30 min.
How do you test for peroxidase?
2:405:02Peroxidase Activity Assay - YouTubeYouTubeStart of suggested clipEnd of suggested clipAdd the measured amount of distilled water into the juice container of the grinder or blender. IntoMoreAdd the measured amount of distilled water into the juice container of the grinder or blender. Into this container put zero minute sample grind it repeat it for remaining five samples.
How do you calculate the ascorbate peroxidase activity?
The enzyme activity can be calculated according to the following equation: Enzyme activity (Units/L) = (ΔAbs × Total assay volume) / ( Δt x ε x l x Enzyme sample volume). extinction coefficient of substrates in units of M-1 cm-1), and l is the cuvette diameter (1cm).
How do you calculate catalase activity from absorbance?
To do the calculations: (Decrease in absorbance x 100/1 ) divided by protein amount in mg divided by time in min=units/mg protein/min. I hope this helps.
How is superoxide dismutase activity measured?
To measure SOD activity, add 100 μl of sample (2–4 mg/ml of cell supernatant or 1–3 mg/ml of the tissue homogenate) or 100 μl of SOD standard sample to the reaction mixture in the presence and absence of 1‐mM cyanide (to measure Mn‐SOD and Total‐SOD activities, respectively).
What is the function of peroxidase in phagocytes?
Peroxidases, enzymes that catalyze the oxidation of certain organic compounds (notably benzidine derivatives) by peroxide, can be demonstrated in many cells of the mononuclear phagocyte series. The distribution and intensity of peroxidase activity, however, varies with stages of cell maturation and phagocytic activity (for review see ref. 1 ). Promonocytes synthesize peroxidase and store the enzyme in cytoplasmic granules, primary lysosomes. Enzyme production generally ceases at the end of the promonocyte stage. The number of granules decrease as promonocytes differentiate into monocytes and in turn distribute granules to mitotic daughter cells. Peroxidase activity may also change with phagocytosis. Peroxidase granules can fuse with phagosomes and release enzyme. Since enzyme synthesis is restricted to promonocytes, phagocytizing monocytes eventually lose their peroxidase content.
What is a POD?
This chapter elaborates about peroxidases (POD), which catalyses the dehydrogenation of a large number of organic compounds, such as phenols and aromatic amines, hydroquinones, and hydroquinoid amines, especially benzidine derivatives. With few exceptions the typical POD belonging to group are hemoproteins. Hemoprotein POD occur in animals, in higher plants, for example, horse radish, pineapples, figs, potatoes, legumes, corn, root vegetables, tobacco plants, in yeasts, in molds, and in bacteria. POD can be determined by the decrease of H2 O 2 or the hydrogen donor or the formation of the oxidized compound. A new POD-substrate that has recently achieved considerable importance for the determination of blood glucose is 2,2′-azino-di- [3-ethyl-benzothiazoline- (6)-sulfonic acid]. The pH optima of different POD with guaiacol as hydrogen donor are not the same. The extinction maximum of guaiacol dehydrogenation product (GDHP) is at 418 nm. The kinetics of the various POD is not uniform. For accurate assays it is important to ensure that removal of blood from the samples is as complete as possible because of the POD activity of leucocytes and the pseudoperoxidative action of hemoglobin in erythrocytes.
How to measure peroxidase activity?
To measure the peroxidase activity the oxidation product or the unchanged substrate is determined chemically or spectrophotometrically at different reaction times. It should be noted that, in the absence of peroxidase, the oxidation of certain organic compounds can be catalysed by H 2 O 2 and traces of heavy metals.
Why is yeast cytochrome C peroxidase used as a sensitive indicator of hydrogen peroxide?
Yeast cytochrome c peroxidase has been effectively used as a sensitive indicator of hydrogen peroxide because of its high affinity for hydrogen peroxide and the high stability of its peroxide compound. View chapter Purchase book. Read full chapter.
How to determine peroxidase?
Peroxidase is determined according to Kaplow (10 ). Air-dried preparations are fixed in 10% formalin in absolute alcohol for 1 min, rinsed in tap water, and dried. Kaplow's reagent, composed of 0.3% (w/v) benzidine dihydrochloride and 0.02% (v/v) hydrogen peroxide (pH 6.0), is poured over the slides and washed off with tap water after 30 sec. The preparations are then dried, counterstained for 6 min with Giemsa stain, washed, and dried. Blood smears are counterstained with Giemsa for 20 min. If staining for peroxidase cannot be performed immediately, the preparations should be kept in the dark.
What is the most likely contribution of DyPs and other low redox potential peroxidases?
DyP has low activity on high redox potential substrates such as lignin model compounds, and therefore, the most likely contribution of DyPs and other low redox potential peroxidases is probably in the oxidative degradation of phenolic residues and modification of lignin-derived compounds in soil.
How many oxidation states does CCP have?
CcP has three accessible heme oxidation states, the most stable under ambient conditions being the resting enzyme ferric state (heme iron=+3), followed in order of decreasing ambient stability by the intermediate ferryl (iron=+4), and the ferrous (iron=+2) states. In addition to differing oxidation states differing ligation states are also encountered for the heme iron at the enzyme's active site. Combined with the two most common heme iron oxidation states found for cytochrome c (Fe +2; Fe +3) one readily sees that there are twelve possible 1:1 combinations of CcP:cytochrome c pairs for each species of cytochrome c ( Table I ). Only stoichiometric 1:1 complexes will be considered here since there is ample direct spectroscopic evidence, as well as kinetic evidence, that 1:1 complexes predominate in solutions of varying protein concentrations between ∼100 micromolar and ∼2 mM ( 10, 13, 14, 17 ).
How many somatic antigens are there in a single capsular type?
The use of a tube agglutination test ( Namioka & Murata, 1961a) first demonstrated that a single capsular type could have two somatic antigens. The somatic antigen typing scheme of Namioka and Murata (1961b) recognized six groups, which offered a rather restricted differentiation. Further differentiation of somatic serotypes of P. multocida became possible with an agar gel diffusion precipitin test ( Heddleston, Gallagher, & Rebers, 1972) which now identifies 16 somatic antigens (designated 1–16). In P. multocida, LPS is an antigen of critical importance for homologous protection stimulated by bacterin vaccines.
How to determine MNP activity?
MnP activity was determined by using 2,6-dimethoxyphenol as a substrate at 470 nm and one unit of enzyme activity is defined as 1 μmol products produced in one minute ( 22 ). Enzyme was added to a solution containing 1.0 mM 2,6-dimethoxyphenol and 1.0 mM MnSO 4 in 50 mM malonate buffer (pH 4.5) and the reaction was initiated with hydrogen peroxide (0.2 mM) in a final volume of 1 ml. As control, hydrogen peroxide (0.2 mM) was replaced by same volume of water. Lignin peroxidase activity was estimated by measuring the amount of veratryl aldehyde formed from 0.1 mM veratryl alcohol (VA) in 50 mM succinate buffer (pH 3.0) containing 0.2 mM hydrogen peroxide (4 ). Laccase activity was measured by monitoring the oxidation of 2,6-dimethoxyphenol at 470 nm in 0.1 M phosphate buffer (pH 6.0) ( 4 ). One unit of enzyme activity is defined as 1 μmol products produced in one minute.
What is the limiting factor for SCN?
The major limiting factor for SCN − peroxidation in human saliva is the availability of H 2 O 2 ( Månsson-Rahemtulla et al., 1983; Tenovuo et al., 1981 ), but concentrations of SCN − below 0.6 mM may also be limiting ( Pruitt et al., 1982 ), and in human milk, the concentrations of SCN − are usually below this level. The low peroxidase concentration in human milk also may be a limiting factor ( Pruitt and Kamau, 1991; Pruitt et al., 1991 ). Thiocyanate ion is secreted by salivary, mammary, lacrimal, and gastric glands and can originate from several sources, but the major source of SCN − is the detoxification of cyanide (CN −) primarily in the liver by thiosulfate–cyanide sulfurtransferase, yielding nontoxic SCN −. Thiocyanate is found in saliva (0.35 –1.24 mM, increasing to 1.38–2.74 mM in smokers) ( Tenovuo, 1985) and milk (0.021–0.122 mM) ( Pruitt and Kamau, 1991 ).
What is the inactivation mechanism of MnP?
MnP activity is dependent on H 2 O 2, but it is also inactivated by H 2 O 2. Oxidation of the native enzyme by 1 equivalent H 2 O 2 forms the active Compound I ( 7) that subsequently oxidizes Mn (II) or a phenolic substrate. The Compound II formed in this process is reduced back to the native enzyme, oxidizing an additional Mn (II) to Mn (III). Mn (II) is a mandatory substrate for Compound II ( 7 ). When excess concentrations of H 2 O 2 are present, native MnP is directly converted to Compound HI that is an inactive form of the enzyme. Compound III can also be formed from Compound II in the presence of excess H 2 O 2 ( 7 ). The inactivation of MnP by high concentrations of H 2 O 2 is one of the major obstacles in applying MnP in pulp bleaching. The understanding of the inactivation mechanism, especially the role of various metal ions in the inactivation, would greatly facilitate the development of a MnP-based pulp bleaching technique.
Why is MnP inactivated?
In fact, it has yet to be demonstrated that the formation of Compound III is the only reason for inactivation of MnP. It is well known that active oxygen species such as hydroxyl free radical formed by the reaction of transition metal ions with H 2 O 2 have detrimental effects on the stability of proteins. Some metal ions may compete with the Fe ligand in heme resulting in the inactivation of the enzyme. According to our results, Mn (II) stabilizes the enzyme in the presence of excess H 2 O 2. This conclusion is also in concert with the data in a recent paper ( 11 ). The stabilization of MnP by Mn (II) is enhanced by malonate. The reason for this stabilization is that Mn (II) competes with H 2 O 2 for Compound II, thereby preventing the formation of the inactive Compound III. Malonate chelates and removes Mn (III) from the active site of MnP, thereby accelerating the catalytic cycle ( 12 ). In this way, Mn (II) competes more efficiently with H 2 O 2 for Compound II. However, Mn (II) cannot stabilize MnP in the presence of an unbleached pulp.
What is the name of the enzyme that catalyzes the oxidation of a number of substrates?
Peroxidase is a hemoprotein catalyzing the oxidation of a number of substrates by hydrogen peroxide:H2O2+substrate−H2→substrate+2H2O
What is the function of peroxidase?
Peroxidases protect mucosal surfaces from microorganisms by catalyzing the peroxidation of halides (Cl −, Br −, I −) and the pseudohalide thiocyanate (SCN −) to generate reactive products that have potent antimicrobial properties. MPO and EPO catalyze the peroxidation of Cl −, Br −, I −, and SCN −, but LPO and SPO do not catalyze the peroxidation of Cl −. In the absence of halides or SCN −, peroxidases behave as catalases and degrade H 2 O 2 to water and oxygen. The catalase and peroxidase activities of these enzymes thereby protect mucosal surfaces by preventing the accumulation of toxic products of oxygen reduction.
What is the function of peroxidases?
Human peroxidases can be divided into two groups: (1) True peroxidases are enzymes whose main function is to generate free radicals in the peroxidase cycle and ...
What is the activity of peroxidase?
Peroxidase Activity of Human Hemoproteins: Keeping the Fire under Control. The heme in the active center of peroxidases reacts with hydrogen peroxide to form highly reactive intermediates, which then oxidize simple substances called peroxidase substrates.
What are the two groups of peroxidases?
Human peroxidases can be divided into two groups: (1) True peroxidases are enzymes whose main function is to generate f …. The heme in the active center of peroxidases reacts with hydrogen peroxide to form highly reactive intermediates, which then oxidize simple substances called peroxidase substrates. Human peroxidases can be divided ...
Is myoglobin a peroxidase?
Hemoglobin, myoglobin, cytochrome c/cardiolipin complexes and cytoglobin are considered as pseudo-peroxidases. Рeroxidases play an important role in innate immunity and in a number of physiologically important processes like apoptosis and cell signaling.
Why is peroxidase important?
Additionally, because peroxidases are relatively stable enzymes, they can serve as an indicator for the efficacy of enzyme inactivating treatments in grains and flours (heat treatment, chemical treatment, etc.)
What enzyme can catalyze the cross-linking of phenolic residues by H2O2?
Peroxidase is an enzyme that can catalyze the cross-linking of phenolic residues by H2O2 resulting in undesirable color changes (graying) and the potential generation of off-flavors in a food product. Determining the average level of peroxidase in different cultivars can help guide breeding decisions.
What is the pH of Bradford method?
Samples are homogenized and enzymes are extracted into a cold, pH 6.5 buffer. A protein determination by the Bradford method is performed on the enzyme extract (supernatant) to normalize subsequent enzyme results to the efficiency of the protein extraction. The enzyme extract is mixed with hydrogen peroxide, guaiacol, and calcium. Peroxidase activity is measured spectrophotometrically by the rate of formation of tetraguaiacol at 470nm.
Why do we use cookies?
However, you can choose not to allow certain types of cookies, which may impact your experience on the site and the services we are able to offer. Click on the different category headings below to find out more and change the default settings according to your preference. You cannot opt out of our Strictly Necessary Cookies because they are deployed in order to ensure the proper functioning of our website.
What are targeted cookies?
Targeting cookies are used by parties that are involved in serving other companies’ advertising on our sites, or that are involved in determining which of our advertisements (or other parties’ advertisements) to show you on third-party websites. These cookies collect information about your online activities, such as the advertisements you have seen or the websites or pages you have visited, in order to draw inferences about what advertising might be relevant to you. This information is then used to show you targeted advertising (sometimes referred to as “interest based advertising” or “online behavioral advertising”) that may be most relevant to you, including when you visit other websites not belonging to us. If these cookies are blocked or disabled, our Site may become less functional, including the potential that some of our services may no longer be available to you.
What are functional cookies?
Functional cookies allow our Site to remember information, such as your preferred language, that can be used to customize your experience on our Site. These cookies also can be used to provide you with information specific to your region, and may assist you in customizing our Site’s appearance.
Most recent answer
Hi!, try this one: dispense in 800 µl of guaiacol buffer (dissolve 40 mM guaiacol in sodium acetate buffer 100 mM at pH 6.0 [dissolve in 80 ml dH2O, 1.28 g di sodium acetate trihydrate, add 50 µl of glacial acetic acid and adjust the pH solution to 6.0 with HCl or NaOH 0.1 M and then fill up to 100 ml]), 100 µl of enzyme extract and incubate for 1-2 min.
Popular Answers (1)
POX catalyses the dehydrogenation of a large number of organic compounds as phenols and aromatic amines. It was determined following the dehydrogenation of guaiacol as a substrate according to Malik and Singh (1980).
Similar questions and discussions
How do I calculate ascorbate peroxidase activity without knowing protein concentration?
