
What is pre-preparative TLC used for?
Preparative TLC separates compounds on a macro scale (0.05–1 g) by utilizing a thick layer of adsorbent (0.5–5 mm). It is used to isolate carbohydrates as pure compounds in sufficient quantities to allow investigations of their properties.
Can TLC be used to run preparative-scale purifications?
Running purifications is one of the main applications of thin layer chromatography. But we can actually apply TLC to run preparative-scale purification. Not just to check how the different compounds/spots on a mixture separate, but to separate our reaction mixtures themselves, and isolate miligrams of pure products!
What is TLC used for in chromatography?
Preparative thin-layer chromatography (tlc) separates compounds on a macro scale (0.05–1 g) by utilizing a thick layer of adsorbent (0.5–5 mm). It is used to isolate carbohydrates as pure compounds in sufficient quantities to allow investigations of their properties 1, 2, 3, 4, 5, 6, 7, 8, 9.
What instruments do I need for preparative TLC?
For preparative TLC, you dont need many instruments. Just you need to have larger TLC plate. After development, you will have to scratch the area of silica adsorbent on which desired compound shows its presence.
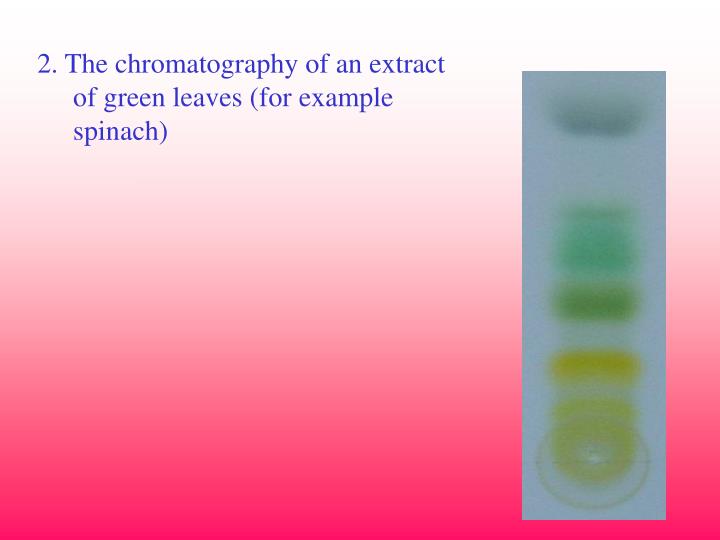
Is TLC preparative or analytical?
analytical toolTLC is an analytical tool widely used because of its simplicity, relative low cost, high sensitivity, and speed of separation. TLC functions on the same principle as all chromatography: a compound will have different affinities for the mobile and stationary phases, and this affects the speed at which it migrates.
What is the difference between preparative separation and analytical separation?
In analytical scale separations the quantity of samples injected is small, usually in micro liter levels whereas in preparative scale separations injection volumes are large so as to yield pure compounds in milligrams to gram levels.
What is prep chromatography?
In practical terms, preparative chromatography generally means the isolation of significant quantities of material (> 0.1 g) using large dimension columns in column chromatography (GC, LC, SFC) or thick-bed plates in TLC.
How many types of TLC are there?
SiliCycle offers different types of plates for thin layer chromatography applications: classical TLC, high performance TLC (also called HPTLC) and preparative TLC (PLC). The plate types are selected based on the type of analysis required and the available budget.
What is a preparative technique?
• A set of devices used prior to isotopic analyses to enable the separation of gaseous species or the in-situ analysis of geological samples. • A laser microdissection instrument to extract specific cells for a total amplification of their genome.
What is the difference between analytical and preparative column?
In the analytical scenario, it is important to identify as many constituents of a sample as possible and/or to determine their concentration, whereas in the preparative case often only one or a few products with a specific purity requirement needs to be obtained.
What is the use of preparative HPLC?
HPLC is used to separate and refine high-purity target compounds from a mixed solution after a synthesis reaction or from natural extracts.
What is preparative purification?
Preparative (Prep) or purification chromatography is defined as the process of using chromatography to isolate a compound in a quantity and at a purity level sufficient for further experiments or processes.
What is preparative column?
Prep Columns For Scaling Up Analytical HPLC Reversed Phase Prep columns offer additional selectivities with fully-porous particles in dimensions that are scalable from 4.6mm to 50mm ID to maximize yield in bulk purification processes.
Why silica gel is used in TLC?
The Silica Gel G for Thin Layer Chromatography as binder is most extensively used as adsorbent in thin layer chromatography because of its excellent separation properties.
Why is acetic acid used in TLC?
Mostly little quantity of acids like formic and acetic acids are added during TLC to reduce tailing.
Is silica polar or nonpolar?
polar compoundReminder: silica is a polar compound. Usually the solvent used to develop the plate is somewhat nonpolar, but choosing an appropriate solvent (or mixture of solvents) is, in general, a trial and error process.
What is preparative separation?
Preparative Separations. A natural extension of SFC is as a preparative separation technique. The column capacity is a function of the packing material and is essentially equivalent to liquid chromatographic systems. Consequently, scale-up procedures are similar. A major advantage for SFC is the solvent.
What is difference between HPLC and preparative HPLC?
1. Analytical HPLC is used for the sample components analysis, to know each purity and content in sample. 2. Preparative HPLC is used to separate each components to get the single components.
What is difference between guard and analytical columns?
Usually HPLC has a guard column ahead of the analytical column to protect and extend the life of the analytical column. The guard column removes particulate matter, contaminants, and molecules that bind irreversibly to the column. The guard column has a stationary phase similar to the analytical column.
Is column chromatography an analytical or a preparatory technique?
Column Chromatography is a preparative technique used to purify compounds depending on their polarity or hydrophobicity. In column chromatography, a mixture of molecules is separated based on their differentials partitioning between a mobile phase and a stationary phase.
How to narrow a TLC band?
It is useful at this point to narrow the sample band by running a polar solvent (typically Et 2 O or EtOAc) up the plate to the upper edge of the band, and then evaporating the solvent (this should be repeated as needed, typically 3 times). Then in a dry chamber prepare about 100 mL of eluent. The ideal eluent should give your compound an Rf of around 0.1 on regular TLC. This will allow multiple elutions if the first one doesn't separate well enough.
What solvent should I use for a crude sample?
Prepare a relatively concentrated solution of your crude sample (~1-2 mL) in a fairly low-boiling solvent (i.e. DCM or Et 2 O). Warning: too large a volume will require multiple applications which broadens the band.
What is preparative TLC?
Well, preparative TLC is just a regular thin layer chromatography separation, but with a bigger plate!
Why is TLC important?
As you can see, TLC is extremely important for both reaction monitoring and product purification, the two cornerstones of any synthesis laboratory.
How to spot TLC?
Spot the TLC mixtures at the corresponding mark in the line above the bottom of the plate. Then elute the plate and see how many compounds there is in your mixture, and how polar are they, just by checking out the different spots. Try to spot your mixtures as tightly as possible. Make very small spots of sample.
What is TLC in chemistry?
If there is one technique or experiment that every chemist, or student learning chemistry should know, it is Thin Layer Chromatography (or TLC for short). If you want to become a synthetic chemist, or you are planning to ace an experimental course on organic chemistry, TLC is something you really need to master. TLC of black ink.
How high is a thin layer chromatography plate?
Usually, a thin layer chromatography plate is around 5–7 cm high, and a line is drawn around 0.5–1.0 cm from the bottom. That is the line in which you will spot your mixtures to separate. It is important that you spot the mixtures above the solvent level on your elution chamber!
What is the stationary phase of TLC?
In TLC, we use a stationary phase (most frequently silica gel ) which is deposited over a glass or aluminum support. We then can spot mixtures of compounds over the same line. Then we elute the TLC with an organic solvent, and the different compounds will move upwards at different rates, allowing the separation of the different components.
What is the oldest technique for chromatography?
Chromatography can get very complex, with complicated and expensive instruments such as GC-MS or HPLC, but the most basic, most important and oldest technique is thin layer chromatography, or TLC.
What is purification by liquid chromatography?
What is Purification by Preparative Liquid Chromatography? 1. Definition. There are many different purification techniques: distillation, crystallization, filtration, … and chromatography. They all have the same goal purify and recover samples, whether an organic or biochemistry synthesis step. Purification by liquid chromatography is always ...
Can TLC be used for purification?
The TLC can be used as a predictive tool for purification method but users have to take care of the adsorbent features differences between the plate and the column, the difference of the eluent migration capillarity for TLC vs. dynamic for LC, the TLC silica binder.
What is the purpose of preparation TLC?
Preparative TLC is used for analytical separations of larger quantities of materials. Because sample mass loading capacity is proportional to the square root of the sorbent layer thickness, thicker layers are used (>250μm up to 1 mm). When a binder is required, the softer inorganic binders (like calcium sulphate) are used so the sample bands can be easily removed. The compounds to be separated are often applied as long streaks, developed and then recovered by scraping the adsorbent from the plate and eluting with a strong solvent.
Does Camag have automatic coaters?
at www.camag.com they have manuel and automated TLC place coaters.
How many spots does EDC have on a TLC?
The reaction of the carboxylic acid and EDC alone gives three spots on a TLC. Are the three spots due to N-O displacement?
Do you need a centrifuge for preparative TLC?
For preparative TLC, you dont need many instruments. Just you need to have larger TLC plate. After development, you will have to scratch the area of silica adsorbent on which desired compound shows its presence. If you don't have centrifuge, you can try removing silica with simple filtration. No doubts, yields will be effected but you may get pure desired compound.
What are the requirements for preparative HPLC?
The requirements of a preparative HPLC system differ according to the quantity of product to be collected, and the nature and number of samples to be purified. However it should provide reliable and complete automation for unattended operations with error-handling methods, and total traceability of all events, including the collected fractions. Fraction collectors are key instruments in preparative HPLC, and should be selected according to their collection facilities and fractionation software. Devices exist that can perform peak collection with automatic tracking of the baseline drift. With such instruments, collection is initiated if the detector signal is above user-set parameters in relation to threshold level and peak width. The graphic sample tracking which is feasible with some recent centralized software is also an important feature in preparative chromatography. Indeed, it can provide useful information to the user on sample identification, collection positions and associated chromatograms.
What is prep chromatography?
Preparative chromatography (Prep) is a unit operation that is underutilized for the purification of active pharmaceutical ingredients. This technique is over 100 years old, and significant improvement has occurred in the past 20 years that makes this technology a very efficient and cost-effective process for either product purification or chiral separations. The author discusses the different modes of chromatography for large-scale separations (Batch, bio, SFC, SMB) and presents strategies for the development of a process in the perspective of commercial-scale manufacturing. The chapter also provides a review of the equipment available for the separation as well as the downstream processing. Finally, the Prep at commercial scale is discussed with an overview of the continuous chromatography process including regulatory aspects, manufacturing point of view, and economic perspectives.
How does linear elution chromatography work?
Linear elution chromatography does not use the full capacity of either the stationary or the mobile phase. Whenever the sample concentrations are in the nonlinear range of the respective adsorption isotherms, the sample molecules compete for the binding sites on the chromatographic surface and interfere with each other's migration. In the case of a single component Langmuirian isotherm, the zone will have an increasingly sharp front and a diffuse rear boundary or “tail” due to the self-interference of the molecules (triangular zones). A simple procedure to maximize the throughput in elution chromatography (“overloaded” elution chromatography), while still maintaining baseline separation, is the so-called touching-band optimization. The sample load is increased until the second component just touches the rear of the first. If the column is severely overloaded, separation becomes largely a matter of the sample composition. In certain cases, the competition of the two components will cause the zone of the less strongly bound component to be pushed ahead by the zone of the more strongly bound one; “sample displacement” occurs often with some felicitous results on the separation. The opposite, a “tag-along” effect, may also be observed. Especially in the case of smaller and chemically/mechanically more stable molecules, a solution to the optimization of the throughput may be to operate the column under conditions of pronounced overloading and simply recycle the mixed zone, i.e., add it to the fresh feed.
How does preparative chromatography support drug discovery?
The impact of preparative chromatography support a drug candidate's lifecycle is felt from Discovery's candidate seeking stage through the development and manufacture of materials for clinical supply, through the launch of a drug candidate into the marketplace . Preparative chromatography is the process of using liquid or supercritical fluids to isolate a sufficient amount of material for other experimental or functional purposes.69 This section describes the use of preparative chromatography in isolating up to milligram quantities of unknown compound (s) for the purpose of structure elucidation by spectroscopic techniques, which is often referred to as semipreparative HPLC and semipreparative SFC. 70–72 This section will focus primarily on preparative methods with the following parameters:
What are the disadvantages of preparative chromatography?
The clear disadvantage of this approach is the large demand on time and materials. A lot of extra equipment is required—HPLC equipment, fraction collectors, vacuum drying apparatus, along with a requirement for a larger amount of DOM material. The time required can increase to a great extent as well, as the DOM is typically separated into at least four fractions [66], or even hundreds of fractions in some studies led by Stenson [11, 61, 67]. This tends to restrict preparative chromatography approaches to the detailed study of a small sample set, and thus is not suited to studies across large geographical regions or timescales.
Who wrote Principles and Practice of Modern Chromatographic Methods?
K. Robards, ... P.E. Jackson, in Principles and Practice of Modern Chromatographic Methods, 2004
What is the best generic method for the purification of small drugs and valuable chemical components at the 10 answer?
Preparative chromatography is today the best generic method for the purification of small drugs and valuable chemical components at the <10 kg-level.
What is TLC used for?
TLC is extremely useful in Biochemical analysis such as separation or isolation of biochemical metabolites from its blood plasma, urine, body fluids, serum, etc. Thin layer chromatography can be used to identify natural products like essential oils or volatile oil, fixed oil, glycosides, waxes, alkaloids, etc.
How to apply sample spots in TLC?
To apply sample spots, thin marks are made at the bottom of the plate with the help of a pencil. Apply sample solutions to the marked spots. Pour the mobile phase into the TLC chamber and to maintain equal humidity, place a moistened filter paper in the mobile phase. ...
How does thin layer chromatography work?
The separation relies on the relative affinity of compounds towards both the phases. The compounds in the mobile phase move over the surface of the stationary phase. The movement occurs in such a way that the compounds which have a higher affinity to the stationary phase move slowly while the other compounds travel fast. Therefore, the separation of the mixture is attained. On completion of the separation process, the individual components from the mixture appear as spots at respective levels on the plates. Their character and nature are identified by suitable detection techniques.
How to maintain humidity in a TLC chamber?
Pour the mobile phase into the TLC chamber and to maintain equal humidity, place a moistened filter paper in the mobile phase.
Which phase of chromatography is particulate free?
Thin Layer Chromatography Mobile phase – Mobile phase is the one that moves and consists of a solvent mixture or a solvent. This phase should be particulate-free. The higher the quality of purity the development of spots is better.
Can you use TLC for a chromatogram?
The detection limit is high and therefore if you want a lower detection limit, you cannot use TLC. It is only a qualitative analysis technique and not quantitative.
Is a qualitative analysis technique quantitative?
It is only a qualitative analysis technique and not quantitative.
