
What is the rocket immunoelectrophoresis method?
The method, originally introduced by Laurell (1) involves a comparison of the sample of unknown concentratio … Rocket immunoelectrophoresis (also referred to as electroimmunoassay) is a simple, quick, and reproducible method for determining the concentration of a specific protein in a protein mixture.
What is immunoelectrophoresis in microbiology?
Immunoelectrophoresis is a powerful analytical technique with high resolving power as it combines separation of antigens by electrophoresis with immunodiffusion against an antiserum. The main advantage of immunoelectrophoresis is that a number of antigens can be identified in serum.
What is the best book on rocket immunoelectrophoresis?
Walker JM (1984). Rocket immunoelectrophoresis. Methods Mol Biol. (1): 317-23 Lydyard, P.M., Whelan,A.,& Fanger,M.W. (2005).Immunology (2 ed.).London: BIOS Scientific Publishers. Parija S.C. (2012).
What type of agarose is used for protein immunoprecipitation?
Agarose of 1% gel slabs of about 1 mm thickness made on buffer with high pH (around 8.6) is usually used for the process but it may vary according to kit. The agarose were choosed as it provides passage of protein through the pore, but provides an anchor for the immunoprecipitates of protein and specific antibodies.
What is Rocket immunoelectrophoresis and give its principle?
Principle: In Rocket Immunoelectrophoresis, negatively charged antigen samples are electrophoresed in an agarose gel containing antibody which is specific to that antigen. As the antigen moves out of the well and enters the agarose gel, it combines with the antibody to form immune complex which is visible as white.
What does immunoelectrophoresis mean?
: electrophoretic separation of proteins followed by identification by the formation of precipitates through specific immunologic reactions.
Is Rocket immunoelectrophoresis qualitative?
Abstract. The rocket immunoelectrophoresis (RIE) test was used for the qualitative detection and quantitative estimation of infectious bursal disease virus (IBDV) specific antigen in experimentally infected chickens and samples collected from suspected outbreaks.
What are the applications of immunoelectrophoresis?
Applications of Immunoelectrophoresis Immunoelectrophoresis is used in patients with suspected monoclonal and polyclonal gammopathies. The method is used to detect normal as well as abnormal proteins, such as myeloma proteins in human serum. Used to analyze complex protein mixtures containing different antigens.
What is rocket electrophoresis?
Rocket electrophoresis (also referred to as electroimmunoassay or electroimmunodiffusion) is a simple, quick, and reproducible method for determining the concentration of a specific protein in a protein mixture.
What are the types of immunoelectrophoresis?
Four types of immunoelectrophoresis (IEP) have been used: electroimmunoassay (EIA also called 'rocket' or 'Laurell rocket'), classical IEP, immunofixation electrophoresis (IFE) and immunoprecipitation of proteins after capillary electrophoresis.
How many classes of IG are there?
fiveThe five primary classes of immunoglobulins are IgG, IgM, IgA, IgD, and IgE. These are distinguished by the type of heavy chain found in the molecule.
Which dye is used in immunoelectrophoresis?
An electrophoresis equipment with a horizontal cooling plate was normally recommended for the electrophoresis. Immunoprecipitates may be seen in the wet agarose gel, but are stained with protein stains like Coomassie brilliant blue in the dried gel.
How do you read immunoelectrophoresis?
0:275:50Immunoelectrophoresis (FL-Immuno/59) - YouTubeYouTubeStart of suggested clipEnd of suggested clipThe strategy involved to study such antigens involves two steps first step is resolving this antigenMoreThe strategy involved to study such antigens involves two steps first step is resolving this antigen mixture into separate antigens. This is done by electrophoresis. Second step involves visualizing.
What are the advantages of immunoelectrophoresis?
Advantages of Technique: Immunoelectrophoresis is a powerful analytical technique with high resolving power as it combines the separation of antigens by electrophoresis with immunodiffusion against an antiserum. 2. The main advantage of immunoelectrophoresis is that a number of antigens can be identified in serum.
What antigen means?
(AN-tih-jen) Any substance that causes the body to make an immune response against that substance. Antigens include toxins, chemicals, bacteria, viruses, or other substances that come from outside the body.
What are antigen and antibody?
Antigen vs antibody An antigen is a foreign substance that enters your body. This can include bacteria, viruses, fungi, allergens, venom and other various toxins. An antibody is a protein produced by your immune system to attack and fight off these antigens.
What does an electrophoresis blood test show?
Protein electrophoresis is a test that measures specific proteins in the blood. The test separates proteins in the blood based on their electrical charge. The protein electrophoresis test is often used to find abnormal substances called M proteins.
What test shows multiple myeloma?
Specialized tests, such as fluorescence in situ hybridization (FISH) can analyze myeloma cells to identify gene mutations. Imaging tests. Imaging tests may be recommended to detect bone problems associated with multiple myeloma. Tests may include an X-ray, MRI, CT or positron emission tomography (PET).
What do immunoglobulins do?
Immunoglobulins, also known as antibodies, are glycoprotein molecules produced by plasma cells (white blood cells). They act as a critical part of the immune response by specifically recognizing and binding to particular antigens, such as bacteria or viruses, and aiding in their destruction.
What does antibodies in urine mean?
Immunoglobulin in the urine can result from: An abnormal buildup of proteins in tissues and organs (amyloidosis) Leukemia. Blood cancer called multiple myeloma. Kidney disorders such as IgA nephropathy or IgM nephropathy.
What is the method of immunoelectrophoresis?
This quantitative one dimensional immunoelectrophoresis method involves a comparison of antigen sample ofunknown concentration with a series of dilutions of a known concentration of the antigen and requires a monospecific antibody against the antigen under investigation.
Why does precipitin have no ring?
No precipitin ring observed- this may be due to inactivation of serum (solution: antiserum must be added after proper cooling down), unlabeled filling of antigens in the well (solution: sample should be loaded without spilling), drying of the agarose gel during incubation (enough moist must be maintained in the chamber).
How does antigen migrate?
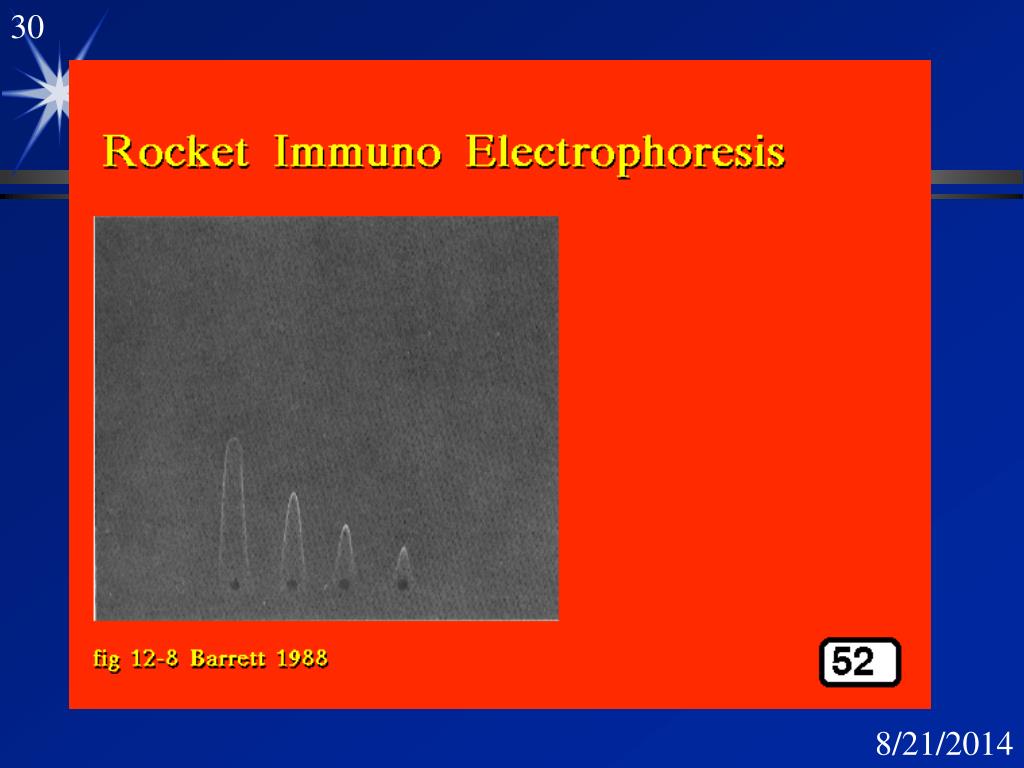
In this method, antigen migrates from the well through agarose gel containing antiserum, forming rocket shaped precipitin peaks. The height of this peak is proportional to the concentration of the antigen loaded in the corresponding well.
Why is agarose used in immunoprecipitation?
The agarose were choosed as it provides passage of protein through the pore, but provides an anchor for the immunoprecipitates of protein and specific antibodies. During the process antigen moves out of the well and enters the agarose gel, it combines with the antibody to form immune complex which is visible as white.
What is a rocket shaped precipitin peak?
Rocket shaped precipitin peaks will be observed in a dark background. It can be marked and measured from the upper edge of the well to the tip of precipitin peaks. It can be used to determine the concentration of unknown antigen by comparing with standard antigens.
How long does it take for agarose gel to set?
Pour agarose gel solution into clean grease free glass plate placed on horizontal surface. Allow the gel to set for about 30 minutes.
How long to incubate TBE buffer?
Do not electrophorese beyond 3 hours, as it is likely to generate heat. Incubate the plate in the moist chamber for 24 hours at 37°C.
What happens when antigens leave the well?
As the protein antigen starts to leave the well and enter the gel, antigen molecules will start to interact, and bind with , antibody molecules. However, at this early stage, there is considerable antigen excess over antibody and no precipitation occurs.
What is rocket electrophoresis?
Rocket electrophoresis (also referred to as electroimmunoassay or electroimmunodiffusion) is a simple, quick, and reproducible method for determining the concentration of a specific protein in a protein mixture. The method, originally introduced by Laurell ( 1 ), involves a comparison of the sample of unknown concentration with a series ...
Where is the majority of antibody-antigen precipitate?
The majority of the antibody-antigen precipitate is indeed at the head of this rocket, but the fine precipitation lines up the side of the rockets are formed by a small amount of antigen diffusing sideways as the antigen passes through the gel.
Is the height of a rocket proportional to the area under the curve?
However, since the rockets are nearly perfect isosceles triangles, the height of the rocket is also proportional to the area under the curve (and hence the antigen concentration), and it is this easier-to-measure parameter that is normally recorded.
Rocket Immunoelectrophoresis Protocol
Electro-immunodiffusion, also known as rocket Immunoelectrophoresis is, is a simple, rapid, and repeatable method for measuring the concentration of antigen in an unknown sample.
Principle
Negatively charged antigen samples are electrophoresed in an agarose gel containing antigen-specific antibody in Rocket Immunoelectrophoresis. As the antigen exits the well and enters the agarose gel, it joins forces with the antibody to create an immunological complex, which appears as white precipitin arcs.
Procedure
Make an agarose gel with 1% agarose. Allow the solution to cool to 50°C before adding the antiserum to the agarose solution. To ensure that the antibody is distributed evenly, mix thoroughly.
Result Interpretation
In a dark background, rocket-shaped precipitin peaks will be visible. It can be marked and measured from the well’s upper edge to the precipitin peaks’ tips. It can be used to compare the concentration of unknown antigens to standard antigens to determine the concentration of unknown antigens.
Applications
In order to diagnose infected bursal. The production of α-amylase and its distribution in diverse seeds during the early stages of germination were investigated.
Limitations
There was no precipitin ring visible, which could be due to serum inactivation, unlabelled antigen filling in the wells, or agarose gel drying during incubation. Due to poor gel pouring and antiserum inactivation, a blur precipitin ring was seen.
What are the advantages of immunoelectrophoresis?
One of the primary advantages of immunoelectrophoresis is its ability to identify a number of antigen in a serum. It is an analytical tool with high resolving capability as it can separate antigens by electrophoresis with immune-diffusion against an antiserum. (1, 6, and 7)
How is immunoelectrophoresis done?
The process of Immunoelectrophoresis is begun by separating the antigen mixture into component parts through the process of electrophoresis and double immuno-diffusion. (1, 2, 3, and 4)
Why are antigens diffused in lateral motion?
They are diffused in a lateral motion for the diffusing antigens to meet allowing the formation of lattice and precipitation, which aids in identifying antigens’ nature. (3, 5, and 7) It was Grabar and Williams who first coined the term immunoelectrophoresis in 1953.
How are antigens reacted?
Once the antigens are successfully separated, they are reacted with particular antiserum placed in troughs with the same distance to the electrophoretic migration and allowed to diffuse.
How long does it take for a gel to run in an electrophoresis chamber?
The gel is then placed in the electrophoresis chamber. The sample is placed on the side of cathode and run for a timeframe of 20 minutes in 100 volts. (2, 5, and 8)
What is agar precipitation?
It is agar’s precipitation under the electric field. As the name suggests, it is a combination of electrophoresis and immune-diffusion. Immunoelectrophoresis is an umbrella term for a number of biochemical procedures to separate and characterize proteins according to electrophoresis and reaction with antibodies.
How long does agarose gel take to dry?
The agarose gel is positioned horizontally and dried using blotter sheets. The gel is soaked in a saline solution for about 10 minutes and dried and washed two times.
How is an antigen mixture separated?
An antigen mixture is first separated into its component parts by electrophoresis and then tested by double immuno-diffusion. Antigens are placed into wells cut in a gel (without antibody) and electrophoresed. A trough is then cut in the gel into which antibodies are placed.
What is the advantage of immunoelectrophoresis?
The main advantage of immunoelectrophoresis is that a number of antigens can be identified in serum.
Why is immunoelectrophoresis limited?
The use of immunoelectrophoresis in food analysis is limited by the availability of specific antibodies.
How long does it take for an antibody to form in a trough?
Antiserum present in the trough moves toward the antigen components resulting in the formation of separate precipitin lines in 18-24 hrs, each indicating reaction between individual proteins with its antibody.
How long does electrophoresis take?
The gel is placed into the electrophoresis chamber with the samples on the cathodic side, and electrophoresis runs for 20 mins/ 100 volts.
When an electric current is applied to a slide layered with gel, the antigen mixture placed in wells is?
When an electric current is applied to a slide layered with gel, the antigen mixture placed in wells is separated into individual antigen components according to their charge and size. Following electrophoresis, the separated antigens are reacted with specific antisera placed in troughs parallel to the electrophoretic migration and diffusion is allowed to occur. Antiserum present in the trough moves toward the antigen components resulting in the formation of separate precipitin lines in 18-24 hrs, each indicating reaction between individual proteins with its antibody.
Which is slower, immunoelectrophoresis or immunofixation?
Immunoelectrophoresis is slower, less sensitive, and more difficult to interpret than Immunofixation electrophoresis.
