What is spectroscopic shift? A hypsochromic shift is the shift of a peak or signal to shorter wavelength (higher energy). Also called a blue shift.
What are chemical shifts in spectroscopy?
Chemical shifts are also used to describe signals in other forms of spectroscopy such as photoemission spectroscopy . Some atomic nuclei possess a magnetic moment ( nuclear spin ), which gives rise to different energy levels and resonance frequencies in a magnetic field.
What is Stokes shift in spectroscopy?
Figure 1 George Gabriel Stokes (1819 to 1903). In fluorescence spectroscopy, the Stokes shift is the difference between the spectral position of the maximum of the first absorption band and the maximum of the fluorescence emission and can be expressed in either wavelength or wavenumber units as shown in Figure 2. 3,4
What is the shift in frequency in NMR spectroscopy?
Chemical Shifts in NMR Spectra. The signal frequency that is detected in nuclear magnetic resonance (NMR) spectroscopy is proportional to the magnetic field applied to the nucleus. This would be a precisely determined frequency if the only magnetic field acting on the nucleus was the externally applied field.
What is meant by chemical shift?
The variations of nuclear magnetic resonance frequencies of the same kind of nucleus, due to variations in the electron distribution, is called the chemical shift.
What is shift in UV spectroscopy?
UV-VIS Terminology Red Shift or Bathochromic Effect: A change in absorbance to a longer wavelength (λ). Auxochrome: A substituent on a chromophore that leads to a red shift. Blue Shift or Hypsochromic Effect: A change in absorbance that leads to a shorter wavelength.
What do you mean by bathochromic shift and hypsochromic shift in UV spectroscopy?
BATHOCHROMIC SHIFT. The shift of absorption to a longer wavelength due to substitution or solvent effect (a red shift). HYPSOCHROMIC SHIFT. The shift of absorption to a shorter wavelength due to substitution or solvent effect (a blue shift).
What do you mean by bathochromic shift?
Bathochromic shift (from Greek βαθύς bathys, "deep"; and χρῶμα chrōma, "color"; hence less common alternate spelling "bathychromic") is a change of spectral band position in the absorption, reflectance, transmittance, or emission spectrum of a molecule to a longer wavelength (lower frequency).
What is blue shift and red shift in spectroscopy?
Redshift and blueshift describe the change in the frequency of a light wave depending on whether an object is moving towards or away from us. When an object is moving away from us, the light from the object is known as redshift, and when an object is moving towards us, the light from the object is known as blueshift.
What is bathochromic shift in UV spectroscopy with example?
Bathochramie Shift or Effect. 2.5) due to the presence of an auxochrome, or solvent effect is called a bathochromic shift or red shift. For example, benzene shows λmax 256 nm and aniline shows λmax 280 nm. Thus, there is a bathochromic shift of 24 nm in the λmax of benzene due to the presence of the auxochrome NH2.
What is the difference between Bathochromic and Hypsochromic?
Bathochromic: a shift of a band to lower energy or longer wavelength (often called a red shift). Hypsochromic: a shift of a band to higher energy or shorter wavelength (often called a blue shift).
What causes Hyperchromic shift?
An increase in the absorbtion of ultraviolet light by a solution of DNA as these molecules are subjected to heat, alkaline conditions, etc. The shift is caused by the disruption of the hydrogen bonds of each DNA duplex to yield single-stranded structures.
What is blue shift in spectroscopy?
blue-shifted absorption and emission spectra. - A spectral shift towards higher wavelengths (i.e. lower energy and lower frequency) is called a red-shift or a bathochromic shift. - A spectral shift towards lower wavelengths (i.e. higher energy and higher frequency) is called a blue-shift or hypsochromic shift.
What is blue shifting?
Key Takeaways. The term "blueshift" refers to the shift in wavelengths of light toward the blue end of the spectrum as an object moves toward us in space. Astronomers use blueshift to understand motions of galaxies toward each other and toward our region of space.
What causes red shift?
A redshift can occur when a light source moves away from an observer, corresponding to the Doppler shift that changes the frequency of sound waves.
What is spectroscopy in science?
Spectroscopy is the study of the interaction between matter and electromagnetic radiation as a function of the wavelength or frequency of the radiation. In simpler terms, spectroscopy is the precise study of color as generalized from visible light ...
How is the spectrum determined in spectroscopy?
In many applications, the spectrum is determined by measuring changes in the intensity or frequency of this energy. The types of radiative energy studied include:
Why is spectroscopy important in chemistry?
Spectroscopy is used in physical and analytical chemistry because atoms and molecules have unique spectra. As a result, these spectra can be used to detect, identify and quantify information about the atoms and molecules. Spectroscopy is also used in astronomy and remote sensing on Earth. Most research telescopes have spectrographs. The measured spectra are used to determine the chemical composition and physical properties of astronomical objects (such as their temperature and velocity ).
What is the first application of atomic spectroscopy?
Atomic spectroscopy was the first application of spectroscopy developed. Atomic absorption spectroscopy and atomic emission spectroscopy involve visible and ultraviolet light. These absorptions and emissions, often referred to as atomic spectral lines, are due to electronic transitions of outer shell electrons as they rise and fall from one electron orbit to another. Atoms also have distinct x-ray spectra that are attributable to the excitation of inner shell electrons to excited states.
What is the term for the measurement of radiation intensity?
Spectroscopy and spectrography are terms used to refer to the measurement of radiation intensity as a function of wavelength and are often used to describe experimental spectroscopic methods. Spectral measurement devices are referred to as spectrometers, spectrophotometers, spectrographs or spectral analyzers .
What is the blackbody spectrum?
A material's blackbody spectrum is a spontaneous emission spectrum determined by its temperature. This feature can be measured in the infrared by instruments such as the atmospheric emitted radiance interferometer. Emission can also be induced by other sources of energy such as flames, sparks, electric arcs or electromagnetic radiation in the case of fluorescence.
What are the applications of spectroscopy?
There are several applications to spectroscopy in the field of medicine, physics, chemistry, and astronomy. Taking advantage of the properties of absorbance, spectroscopy can be used to identify certain states of nature. Such examples include: 1 Cure monitoring of composites using optical fibers. 2 Estimate weathered wood exposure times using near infrared spectroscopy. 3 Measurement of different compounds in food samples by absorption spectroscopy both in visible and infrared spectrum. 4 Measurement of toxic compounds in blood samples 5 Non-destructive elemental analysis by X-ray fluorescence. 6 Electronic structure research with various spectroscopes. 7 Radar to determine the speed and velocity of a distant object 8 Finding the physical properties of a distant star or nearby exoplanet using the Relativistic Doppler effect. 9 In-ovo sexing for chicks
Abstract
We have developed a novel, spectroscopic technique for high-sensitivity, label-free DNA quantification. We demonstrate that an optical resonance (whispering gallery mode) excited in a micron-sized silica sphere can be used to detect and measure nucleic acids. The surface of the silica sphere is chemically modified with oligonucleotides.
Introduction
With genomes of many species completed, we are at the beginning of a revolution in genetic analysis. Technological advances of the recent years have made this revolution possible by replacing labor-intensive, traditional biochemical methods with automated nucleic acid analysis techniques ( Jaklevic et al., 1999; Marshall and Hodgson, 1998 ).
Experimental protocol
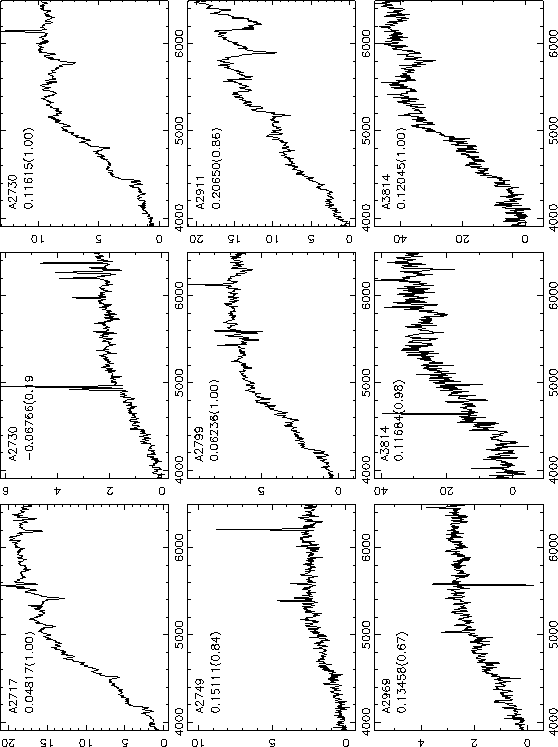
Fig. 1 A shows the schematic of the experiment.
Results
Experiments were performed in a liquid sample cell ( Vollmer et al., 2002) containing two silica spheres immersed in a buffer solution and evanescently coupled ( Griffel et al., 1996; Laine et al., 1999; Serpenguezel et al., 1995) to the same single-mode, near-infrared optical fiber ( Fig. 1 A ).
Discussion
In our measurements, we demonstrate that the perturbation of an optical microsphere resonator can be used for high-sensitivity, label-free DNA quantification. A mass loading of only ∼6 pg/mm 2 of polarizable DNA material on the microsphere surface leads to a detectable spectroscopic shift of the resonance.
What is CSI in physics?
Metabolic imaging (CSI) consists in recording the spectroscopic data for a group of voxels, in slice (s) (2D) or by volume (3D). It is based on a repetition of STEAM or PRESS type sequences to which is added spatial phase encoding. The number and direction of phase encodings depend on the number of dimensions explored (1D, 2D or 3D), adding on to acquisition time. The duration of the sequence is equal to TR ∙ Nph1D ∙ Nph2D ∙ Nph3D ∙ NSA (NphxD number of phase encoding steps in direction x).
Why is fast CSI important?
Fast CSI provides an important speed gain compared to 2D CSI, by performing spatial encoding in one direction during signal acquisition, by means of oscillating gradients, similar to spatial encoding in an echo planar sequence. These techniques are less sensitive than the classic CSI sequence. The speed gain is particularly useful in 3D spectroscopic imaging or in the case of motion artifacts.
What is spectroscopy in science?
What is Spectroscopy? Spectroscopy means the dispersion of light into component colours. In simple words, it is a method to measure how much light is absorbed by a chemical substance and at what intensity of light passes through it.
Which type of spectroscopy deals with the infrared region of the electromagnetic spectrum?
Infrared Spectroscopy: The type of spectroscopy which deals with the infrared region of the electromagnetic spectrum is Infrared Spectroscopy. The rays of the infrared region have longer wavelength whereas having a lower frequency than light. Infrared spectroscopy is based on absorption spectroscopy.
What is the difference between inelastic scattering and resonance spectroscopy?
Inelastic scattering. Coherent or resonance spectroscopy. In Chemistry, Spectroscopy helps to study or analyse various chemical compounds or elements , whereas , in Physics, it helps to determine the makeup of the atmospheres of planets.
Why do lasers shift energy?
The energy of the laser photons shifts up & down due to the interaction of the light with the molecules or phonons of an object. This up down shift of laser photon forms the vibrational modes of an object or system. Raman Spectroscopy Energy Levels.
What is a raman spectroscopic technique?
Raman Spectroscopy: Raman Spectroscopy is a spectroscopic technique which is used to analyze vibrational, rotational, and other low-frequency modes in a system. Raman’s spectroscopy is commonly used in the branch of chemistry to provide a fingerprint by which molecules can be identified.
What is the interaction between light and material?
Type of interaction between light and material: In spectroscopy, the type of interaction between light and the material is usually -: In Chemistry, Spectroscopy helps to study or analyse various chemical compounds or elements, whereas, in Physics, it helps to determine the makeup of the atmospheres of planets.
What is the extent of Stokes shift?
The extent of the Stokes shift depends on the particular fluorophore and its solvation environment, with more polar solvents typically giving larger Stokes shifts . The emission and absorption spectra of two fluorophores with a small and large Stokes shift are shown in Figure 3.
Why is it called the Stokes Shift?
Stokes extensively studied the properties of what we now know as fluorescence and investigated how the properties of the fluorescent light differed from the incident light. He presented his findings “ On the Change of the Refrangibility of Light ” to the Royal Society of London in 1852. 1 In this work he stated that: 1,2
How does anti-Stokes scattering work?
In Stokes Raman scattering the molecule gains a quantum of vibrational energy from the photon during the scattering process and the Stokes radiation, therefore, has a longer wavelength than the incident radiation. In anti-Stokes Raman scattering the reverse occurs, with the molecule losing a quantum of vibrational energy during the scattering process and the anti-Stokes radiation, therefore, has a shorter wavelength than the incident. Raman peaks are characterised by their wavenumber shift away from the incident radiation, with Stokes peaks having a positive wavenumber shift and anti-Stokes shifts being negative.
Which type of scattering has a shorter wavelength?
In anti-Stokes Raman scattering the reverse occurs, with the molecule losing a quantum of vibrational energy during the scattering process and the anti-Stokes radiation, therefore, has a shorter wavelength than the incident.
Which law states that fluorescence emission occurs at a longer wavelength than the incident light?
This is Stokes’ Law and states that the fluorescence emission occurs at a longer wavelength than the incident light. The shift to longer wavelength between the absorption and fluorescence spectra is accordingly called the Stokes shift in his honour.
How to determine intensity of transition?
The intensity of a transition is determined by the population difference between the initial and final states and the transition probability between those states which can be calculated using the Franck-Condon principle.
Is Stokes shift an approximation?
It should be noted that the wavenumber Stokes shift expression written above is actually only an approximation since it assumes that the wavenumber maxima are at the same position as the wavelength maxima which is not strictly true. When fluorescence spectra are converted from a wavelength scale to a wavenumber scale the position ...
What is the difference between a lower chemical shift and a lower magnetic shift?
the signal or shift is downfield or at low field or paramagnetic. Conversely a lower chemical shift is called a diamagnetic shift, and is upfield and more shielded .
What is the chemical shift of the nucleus in the vicinity of an electronegative atom?
A nucleus in the vicinity of an electronegative atom experiences reduced electron density and the nucleus is therefore deshielded. In proton NMR of methyl halides (CH 3 X) the chemical shift of the methyl protons increase in the order I < Br < Cl < F from 2.16 ppm to 4.26 ppm reflecting this trend. In carbon NMR the chemical shift of the carbon nuclei increase in the same order from around −10 ppm to 70 ppm. Also when the electronegative atom is removed further away the effect diminishes until it can be observed no longer.
What is anisotropic induced magnetic field?
Anisotropic induced magnetic field effects are the result of a local induced magnetic field experienced by a nucleus resulting from circulating electrons that can either be paramagnetic when it is parallel to the applied field or diamagnetic when it is opposed to it. It is observed in alkenes where the double bond is oriented perpendicular to the external field with pi electrons likewise circulating at right angles. The induced magnetic field lines are parallel to the external field at the location of the alkene protons which therefore shift downfield to a 4.5 ppm to 7.5 ppm range. The three-dimensional space where a diamagnetic shift is called the shielding zone with a cone-like shape aligned with the external field.
What is the name of the variation in the frequency of a nucleus?
The variations of nuclear magnetic resonance frequencies of the same kind of nucleus, due to variations in the electron distribution, is called the chemical shift. The size of the chemical shift is given with respect to a reference frequency or reference sample (see also chemical shift referencing ), usually a molecule with a barely distorted ...
When was the Knight shift first observed?
The related Knight shift (first reported in 1949) is observed with pure metals. The NMR chemical shift in its present-day meaning first appeared in journals in 1950. Chemical shifts with a different meaning appear in X-ray photoelectron spectroscopy as the shift in atomic core-level energy due to a specific chemical environment. The term is also used in Mössbauer spectroscopy, where similarly to NMR it refers to a shift in peak position due to the local chemical bonding environment. As is the case for NMR the chemical shift reflects the electron density at the atomic nucleus.
What is indirect referencing in NMR?
Indirect referencing uses a channel other than the one of interest to adjust chemical shift scale correctly, i.e. the solvent signal in the deuterium (lock) channel can be used to reference the a 1 H NMR spectrum. Both indirect and direct referencing can be done as three different procedures:
What is gamma spectroscopy?
Gamma spectroscopy is a radionuclide measurement method. While a Geiger counter determines only the count rate, a gamma spectrometer will determine the energy and the count rate of gamma-rays emitted by radioactive substances.
Why is gamma spectroscopy important?
Gamma spectroscopy is an extremely important method. Most radioactive sources produce gamma-rays of various energies and intensities. ADVERTISEMENTS: When these emissions are collected and analyzed with a gamma spectroscopy system, a gamma energy spectrum can be produced.
Why is excitation wavelength important?
The excitation wavelength is matched to an electronic transition of the molecule or crystal, so that vibrational modes associated with the excited electronic state are greatly enhanced. This is useful for studying large molecules such as polypeptides, which might show hundreds of bands in “conventional” Raman spectra. It is also useful for associating normal modes with their observed frequency shifts.
How do X-rays get dispersed?
Typically, the X-rays emerging from a sample must pass a source-defining slit, then optical elements (mirrors and/or gratings) disperse them by diffraction according to their wavelength and, finally, a detector is placed at their focal points.
What are peaks in a gamma ray?
Gamma rays detected in a spectroscopic system produce peaks in the spectrum. These peaks can also be called lines, by analogy to optical spectroscopy. The width of the peaks is determined by the resolution of the detector, a very important characteristic of gamma spectroscopic detectors. Resolution is analogous to Resolving power in optical spectroscopy.
How do scintillation detectors work?
Scintillation detectors use crystals that emit light when gamma-rays interact with the atoms in the crystals. The intensity of the light produced is proportional to the energy deposited in the crystal by the gamma-ray. The mechanism is similar to that in a Thermo-luminescent Dosimeter. The detectors are joined to photomultipliers that convert the light into electrons and amplify the electrical signal provided by the electrons.
Which instrument allowed the use of a single optical element that combines diffraction and focusing?
Henry Augustus Rowland (1848-1901) devised an instrument that allowed the use of a single optical element that combines diffraction and focusing: a spherical grating. Reflectivity of X -rays is low regardless of the used material and, therefore, grazing incidence upon the grating is necessary X-ray beams impinging on a smooth surface at a few degrees glancing angle of incidence undergo external total reflection which is taken advantage of to enhance the instrumental efficiency substantially.
What is the symbol for chemical shift?
In practice the chemical shift is usually indicated by a symbol δ which is defined in terms of a standard reference.
What causes the NMR signal frequency to shift?
This change in the effective field on the nuclear spin causes the NMR signal frequency to shift.

Overview
Classification of methods
Spectroscopy is a sufficiently broad field that many sub-disciplines exist, each with numerous implementations of specific spectroscopic techniques. The various implementations and techniques can be classified in several ways.
The types of spectroscopy are distinguished by the type of radiative energy involved in the interaction. In many applications, the spectrum is determined b…
Introduction
Spectroscopy is a branch of science concerned with the spectra of electromagnetic radiation as a function of its wavelength or frequency measured by spectrographic equipment, and other techniques, in order to obtain information concerning the structure and properties of matter. Spectral measurement devices are referred to as spectrometers, spectrophotometers, spectrographs or spectral analyzers. Most spectroscopic analysis in the laboratory starts with a …
Theory
The central theory of spectroscopy is that light is made of different wavelengths and that each wavelength corresponds to a different frequency. The importance of spectroscopy is centered around the fact that every different element in the periodic table has a unique light spectrum described by the frequencies of light it emits or absorbs consistently appearing in the same part of the electromagnetic spectrum when that light is diffracted. This opened up an entire field of s…
Other types
Other types of spectroscopy are distinguished by specific applications or implementations:
• Acoustic resonance spectroscopy is based on sound waves primarily in the audible and ultrasonic regions.
• Auger electron spectroscopy is a method used to study surfaces of materials on a micro-scale. It is often used in connection with electron microscopy.
Applications
There are several applications of spectroscopy in the fields of medicine, physics, chemistry, and astronomy. Taking advantage of the properties of absorbance and with astronomy emission, spectroscopy can be used to identify certain states of nature. The uses of spectroscopy in so many different fields and for so many different applications has caused specialty scientific subfield…
History
The history of spectroscopy began with Isaac Newton's optics experiments (1666–1672). According to Andrew Fraknoi and David Morrison, "In 1672, in the first paper that he submitted to the Royal Society, Isaac Newton described an experiment in which he permitted sunlight to pass through a small hole and then through a prism. Newton found that sunlight, which looks white to us, is actually made up of a mixture of all the colors of the rainbow." Newton applied the word "s…
See also
• Applied spectroscopy
• Astronomical spectroscopy
• Biomedical spectroscopy
• Coronium
• Frances Lowater