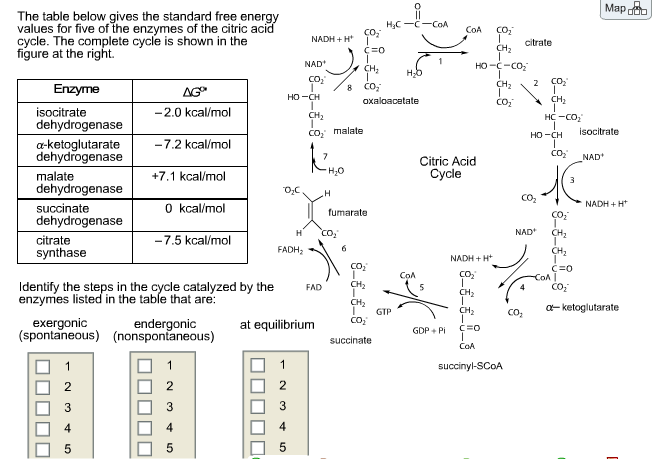
The standard-state free energy of reaction is a measure of how far the standard-state is from equilibrium. But the magnitude of Go depends on the temperature of the reaction. As a result, the equilibrium constant must depend on the temperature of the reaction.
What is the formula for standard free energy?
The standard free energy of formation (ΔG ∘ f), is the change in free energy that occurs when 1 mol of a substance in its standard state is formed from the component elements in their …
How do you calculate free energy?
The standard state free energy of formation of a substance is the difference in the free energy of the substance and the elements which makes it in their thermodynamically stable state. …
How to calculate standard free energy change?
The standard free energy of a substance represents the free energy change associated with the formation of the substance from the elements in their most stable forms as they exist under …
What is standard free energy change?
As might be expected, the standard-state free energy of formation of a substance is the difference between the free energy of the substance and the free energies of its elements in their …
What is meant by standard free energy?
What is the difference between free energy and standard free energy?
What is standard state Delta G?
What is standard free energy in thermodynamics?
What is meant by the standard state condition?
What is difference between ∆ G and ∆ G?
∆G° is at standard conditions (1 atm and 25 degrees Celsius). ∆G° is always the same because it is referring to when the reactants/products are at standard temperature/pressure. As the rxn goes towards equilibrium, ∆G changes because the rxn is proceeding.Feb 15, 2021
Why is Gibbs free energy important?
The importance of the Gibbs function can hardly be over-stated: it serves as the single master variable that determines whether a given chemical change is thermodynamically possible.Feb 20, 2022
What does it mean when Delta G naught is zero?
Is free energy a state function?
Why free energy is called free energy?
What is free energy in terms of thermodynamics class 11?
standard free energy change Definition
Standard free energy is the change in free energy associated with the formation of a substance from its elements in their most stable form under standard conditions.
Overview of Standard Free-Energy Change (G)
The Gibbs free energy was developed by in 1870 by scientist Josiah Gibbs. From second law of thermodynamics under standard conditions of temperature and pressure, a system tries to achieve the minimum Gibbs free energy. The Gibbs free energy is given by the relation,
Standard state free energy in reaction
A reaction is said to be spontaneous if it occurs all by itself for example, in the formation of coal and petroleum from plants and animals. A non-spontaneous process occurs under the action of some external force like the chemical reactions.
Standard free energy in electrochemistry
The equation describes the relationship between cell potential and the standard state cell potential at any instant. E, potential of the cell measured in volts
What is Gibbs free energy?
The Gibbs free energy of a system at any moment in time is defined as the enthalpy of the system minus the product of the temperature times the entropy of the system. G = H - TS. The Gibbs free energy of the system is a state function because it is defined in terms of thermodynamic properties that are state functions.
What does the value of G tell us?
The value of G for a reaction at any moment in time tells us two things. The sign of G tells us in what direction the reaction has to shift to reach equilibrium. The magnitude of G tells us how far the reaction is from equilibrium at that moment.
Why are some reactions spontaneous?
Some reactions are spontaneous because they give off energy in the form of heat ( H < 0). Others are spontaneous because they lead to an increase in the disorder of the system ( S > 0). Calculations of H and S can be used to probe the driving force behind a particular reaction. Practice Problem 5:
What is the standard state of a gas?
Definitions of standard states: For a gas, the standard state is as a pure gaseous substance as a (hypothetical) ideal gas at a pressure of exactly 1 bar. For a substance present in a solution, the standard state is a concentration of exactly 1 M at an applied pressure of 1 bar, but exhibiting infinite-dilution behavior.
What is the standard enthalpy of formation?
The standard enthalpy of formation (ΔH0f) of a compound is the change in enthalpy that accompanies the formation of 1 mole of a compound from its elements with all substances in their standard states. The table below shows the standard enthalpy of formation, the standard Gibbs free energy of formation, standard entropy and molar heat capacity ...
What is the enthalpy of a system?
Enthalpy is a state function, defined by the internal energy (E), the pressure (P) and volume (V) of a system: H = E + PV and ΔH = ΔE + Δ (PV) For enthalpy, there are no method to determine absolute values, only enthalpy changes (ΔH values) can be measured. Then it is important to have a common and well defined reference state.
Can enthalpy be measured?
For enthalpy, there are no method to determine absolute values, only enthalpy changes (ΔH values) can be measured. Then it is important to have a common and well defined reference state. Since enthalpy is a state function, a change in enthalpy does not depend on the pathway between two states.
What does the superscript degree symbol mean?
The superscript degree symbol (°) indicates that substances are in their standard states. (ΔH°, ΔG°, S°.....)
What is standard free energy?
Standard free energy is the free energy defined at standard conditions. • Therefore, standard free energy is given at 298K temperature and 1 atm pressure, but the free energy value can change depending on the temperature and pressure.
What is free energy?
The amount of work that a thermodynamic system can perform is known as free energy. Free energy can be described using two terms, Helmholtz free energy and Gibbs free energy. In chemistry, when we use the word “free energy” that means Gibbs free energy. In physics, free energy refers to Helmholtz free energy.
What is the entropy of heat?
Entropy is related to the amount of heat generated; that is the extent to which energy has been degraded. But, in fact, the amount of extra disorder caused by a given amount of heat q depends on the temperature. If it is already very hot, a bit of extra heat does not create much more disorder, but if the temperature is very low, ...
What is the second law of thermodynamics?
The second law of thermodynamics is related to entropy, and it says, “the entropy of the universe increases in a spontaneous process.”. Entropy is related to the amount of heat generated; that is the extent to which energy has been degraded.
