
Application
- for the nonenzymatic succinylation of lysine in vitro,
- as a substrate for adipic acid biosynthesis in recombinant E. coli
- in isocitrate dehydrogenase (ICDH) treatment for the succinylation of proteins
- as a substrate to study the specificity and kinetics of enzymes such as acetate:succinate CoA-transferase and 5-aminolevulinate synthase (ALA synthase)
What is the role of succinyl CoA in the citric acid cycle?
The citric acid cycle intermediate succinyl-CoA plays an important role in fatty acid and amino acid metabolism because it is the entry point of odd-chain fatty acids, propionate, and the branched chain amino acids valine and isoleucine into the citric acid cycle.
What is succinyl-CoA?
Succinyl-coenzyme A, abbreviated as succinyl-CoA ( / ˌsʌksɪnəlˌkoʊˈeɪ /) or SucCoA, is a thioester of succinic acid and coenzyme A . It is an important intermediate in the citric acid cycle, where it is synthesized from α-ketoglutarate by α-ketoglutarate dehydrogenase through decarboxylation. During the process, coenzyme A is added.
How do you convert succinyl CoA to succinate?
Succinyl-CoA is first converted to malate, and then to pyruvate where it is then transported to the matrix to enter the citric acid cycle. It is converted into succinate through the hydrolytic release of coenzyme A by succinyl-CoA synthetase (succinate thiokinase).
What is the difference between propionyl CoA and succinyl-CoA?
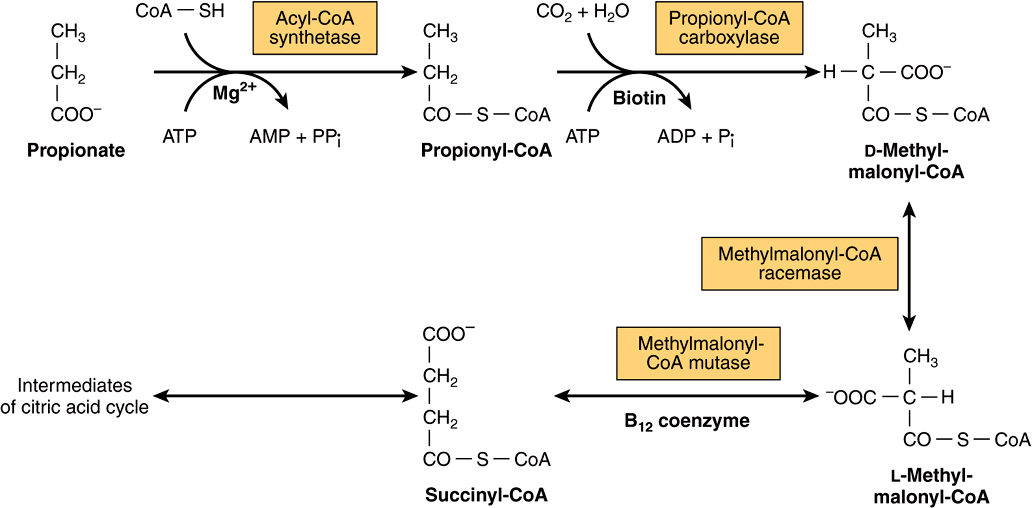
Propionyl-CoA is carboxylated to D-methylmalonyl-CoA, isomerized to L-methylmalonyl-CoA, and rearranged to yield succinyl-CoA via a vitamin B 12 -dependent enzyme. While Succinyl-CoA is an intermediate of the citric acid cycle, it cannot be readily incorporated there because there is no net consumption of Succinyl-CoA.
What happens to succinyl-CoA?
Fate. It is converted into succinate through the hydrolytic release of coenzyme A by succinyl-CoA synthetase (succinate thiokinase). Another fate of succinyl-CoA is porphyrin synthesis, where succinyl-CoA and glycine are combined by ALA synthase to form δ-aminolevulinic acid (dALA).
Is succinyl-CoA used for heme synthesis?
Pathway of Heme Synthesis The building blocks of heme are succinyl CoA and glycine, which combine to form δ-aminolevulinic acid (ALA), the first committed intermediate of the pathway.
Is succinyl-CoA an enzyme?
Succinyl-CoA Synthetase SCS, also known as succinyl CoA ligase (SUCL), is the fifth enzyme of the TCA cycle.
Can succinyl-CoA be used for gluconeogenesis?
Succinyl-CoA is a component of the TCA cycle and is converted to oxaloacetate and then into glucose by gluconeogenesis.
How does succinyl-CoA produce ATP?
Succinyl-CoA synthetase (SCS) is the only mitochondrial enzyme capable of ATP production via substrate level phosphorylation in the absence of oxygen, but it also plays a key role in the citric acid cycle, ketone metabolism and heme synthesis.
Where does succinyl-CoA come from?
Succinyl-CoA is an important intermediate in the citric acid cycle, where it is synthesized from α-Ketoglutarate by α-ketoglutarate dehydrogenase (EC 1.2.
What type of regulator is succinyl-CoA?
Succinyl-CoA synthetase is not a major regulator in the Krebs cycle, making it dependent on the steps prior. However, there has been evidence that a high-affinity GDP-binding site does allosterically regulate the activity of the enzyme.
What enzyme converts succinyl to succinate?
Succinyl-CoA ligase, also called succinate synthase, is an enzyme in the Krebs cycle that converts succinyl-CoA to succinate and free coenzyme A, and converts ADP or guanosine diphosphate (GDP) to ATP or guanosine triphosphate (GTP) respectively (2,3).
What is produced when succinyl-CoA is changed to succinate?
So, the answer is 'ATP in plants and GTP in animals'.
What enzyme is used in glycolysis and gluconeogenesis?
Two key enzymes that regulate irreversible steps in these two processes are pyruvate kinase (PK) and phosphoenolpyruvate carboxy kinase (PEPCK), which catalyze the last and first step of glycolysis and gluconeogenesis, respectively, and are both regulated by lysine acetylation.
What can be used for gluconeogenesis?
The major substrates for gluconeogenesis include lactate, pyruvate, propionate, glycerol, and 18 of the 20 amino acids (the exceptions are leucine and lysine).
Which enzyme is used in gluconeogenesis but not in glycolysis?
Glucose 6-phosphate is dephosphorylated by glucose 6-phosphatase to form glucose, which is free to enter the bloodstream. This reaction is unique to gluconeogenesis and bypasses the irreversible reaction catalyzed by the glycolytic enzyme hexokinase.
What is succinyl-coa?
Succinyl-CoA is a high-energy thiol ester compound with a ΔG0' for hydrolysis of about −33·5 kJ (−8 kcal) mol −1, i. e. of the same order as that required for the synthesis of ATP from ADP. In the course of the cycle, cleavage of the thioester bond is coupled with the phosphorylation of guanosine diphosphate (GDP) and the Δ G0' of the overall reaction, which is reversible and is catalysed by succinyl-CoA synthase, is close to zero.
How is succinyl coa formed?
Succinyl-CoA can also be synthesized from propionyl-CoA by way of methylmalonyl-CoA, which is formed in the oxidation of branched-chain amino acids (e.g., valine, isoleucine) and in the terminal stage of oxidation of odd-chain-length fatty acids ( Chapter 18 ).
What is the SCS enzyme?
SCS, also known as succinyl CoA ligase (SUCL), is the fifth enzyme of the TCA cycle. The protein is a heterodimeric enzyme, the α subunit of which, encoded by the SUCLG1 gene, couples the reversible conversion of succinyl-CoA to succinate bringing to the formation of a nucleoside triphosphate molecule (either GTP or ATP). The β subunit is encoded by either the SUCLG2 or the SUCLA2 gene, determining the specificity for GDP or ADP, respectively.
How does methylmalonyl-CoA convert to L-isomer?
Methylmalonyl-CoA racemase converts D-methylmalonyl-CoA to the L-isomer by labilization of an α -hydrogen atom, followed by uptake of a proton from the medium.
What is the role of succinyl-coa in the citric acid cycle?
The citric acid cycle intermediate succinyl-CoA plays an important role in fatty acid and amino acid metabolism because it is the entry point of odd-chain fatty acids, propionate, and the branched chain amino acids valine and isoleucine into the citric acid cycle.
What is the calibration standard for amino acids?
The amino acids used as calibration standard are prepared in 5% metaphosphoric acid. Amino acids can be detected with a Model 5600 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +600, +700, +800, and +900 mV.
How to isolate SCOT protein?
To isolate the protein, bands (10–100 μ g) corresponding to the carboxy-terminal fragment are cut and placed in 0.5-ml plastic tubes containing 100 μ l of 0.1 M sodium acetate buffer, pH 7.4, mixed with 5% (w/w) pronase from Staphylococcus griseus (Boehringer) to hydrolyze the proteins into amino acids. Hydrolysis is carried out overnight at 50°C. [It can be stopped by the addition of 100 μ l 10% (w/v) metaphosphoric acid.] Samples are then centrifuged at 18,000 g for 20 min, and supernatants are transferred to autosample microvials for injection onto a HPLC column. Amino acids (tyrosine, 3-nitrotyrosine, tryptophan, 4-nitrotryptophan, 5-nitrotryptophan, 5-hydroxytryptophan, and kynurenine) are separated by HPLC, fitted with a Shimadzu class VP solvent delivery system using a reversed-phase C18 Gemini column (4.6 ×150 mm, 5 μ m, Phenomenex). The mobile phase for isocratic elution consists of 25 m M monobasic sodium phosphate, 12.5% methanol, pH 2.7, adjusted with 85% phosphoric acid. The flow rate is 1 ml/min. Under these conditions, separation is completed in 30 min; 5-nitrotryptophan is the last eluted peak, with a retention time of approximately 27 min. For the analysis of tyrosine and 3NT, omit the methanol from the solvent. The elution of 3NT occurs toward the end, with a retention time of approximately 25 min. The amino acids used as calibration standard are prepared in 5% metaphosphoric acid. Amino acids can be detected with a Model 5600 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +600, +700, +800, and +900 mV. With a signal-to-noise ratio of 4:1, the lower limit for electrochemical detection is 300 fmol for 3-nitrotyrosine and 4- and 5-nitrotryptophan and 200 fmol for tyrosine, tryptophan, 5-hydroxytryptophan, and kynurenine. For quantification, inject each sample twice and average the peak areas. Representative HPLC chromatograms are shown Fig. 19.2. The separation of standards (tyrosine, 3NT, tryptophan, 4-and 5-nitrotryptophan) is shown in trace A ( Fig. 19.2 ). Amino acid analysis of the SCOT carboxy-terminal fragment (trace B, Fig. 19.2) indicates the absence of 3NT and 4- and 5-tryptophan. The amino acid nitrohydroxytryptophan (W OH,N with a retention time of 21 min) occurs exclusively in the full-length protein and the C-terminal fragment. The UV absorption spectrum of nitrohydroxytryptophan, detected with a diode array, is similar to those of nitrated aromatic amino acids, with a characteristic emission peak at 340 to 360 nm. The retention time and UV spectrum of nitrohydroxytryptophan observed during HPLC analysis of the digest of the SCOT protein should be identical to the tryptophan derivative produced in vitro (trace C, Fig. 19.2 ).
How is succinyl coa formed?
Succinyl-CoA can also be synthesized from propionyl-CoA by way of methylmalonyl-CoA, which is formed in the oxidation of branched-chain amino acids (e.g., valine, isoleucine) and in the terminal stage of oxidation of odd-chain-length fatty acids (Chapter 18).
What is the source of propionyl-CoA?
Important sources of propionyl-CoA are the catabolism of isoleucine, valine, methionine, and threonine (Chapter 17). Cholesterol side chain oxidation also yields propionyl-CoA. Thus, propionyl-CoA is derived from the catabolism of lipids and proteins. In ruminants, propionate is largely derived from bacterial fermentation in the rumen.
What is the structure of propionyl-coa carboxylase?
Propionyl-CoA carboxylase is a tetramer of nonidentical subunits, α and β. The native enzyme (M.W. ∼540,000) appears to have the structure ( αβ) 4.Biotin is bound through an amide linkage to an ε -amino group of a lysyl residue in the α-subunit. Carboxylation is a two-step reaction similar to that of acetyl-CoA carboxylase (see below). The first step requires ATP and Mg2+ and fixes CO 2 with the formation of an apoenzyme-biotin-CO 2 complex. In the second step, the carboxyl group from the biotinyl complex is transferred to propionyl-CoA to form D- methylmalonyl-CoA.
How does methylmalonyl-CoA convert to L-isomer?
Methylmalonyl-CoA racemase converts D-methylmalonyl-CoA to the L-isomer by labilization of an α -hydrogen atom, followed by uptake of a proton from the medium.
How to isolate SCOT protein?
To isolate the protein, bands (10–100 μg) corresponding to the carboxy-terminal fragment are cut and placed in 0.5-ml plastic tubes containing 100 μ l of 0.1 M sodium acetate buffer, pH 7.4, mixed with 5% (w/w) pronase from Staphylococcus griseus (Boehringer) to hydrolyze the proteins into amino acids. Hydrolysis is carried out overnight at 50°C. [It can be stopped by the addition of 100 μ l 10% (w/v) metaphosphoric acid.] Samples are then centrifuged at 18,000 g for 20 min, and supernatants are transferred to autosample microvials for injection onto a HPLC column. Amino acids (tyrosine, 3-nitrotyrosine, tryptophan, 4-nitrotryptophan, 5-nitrotryptophan, 5-hydroxytryptophan, and kynurenine) are separated by HPLC, fitted with a Shimadzu class VP solvent delivery system using a reversed-phase C18 Gemini column (4.6 ×150 mm, 5 μm, Phenomenex). The mobile phase for isocratic elution consists of 25 m M monobasic sodium phosphate, 12.5% methanol, pH 2.7, adjusted with 85% phosphoric acid. The flow rate is 1 ml/min. Under these conditions, separation is completed in 30 min; 5-nitrotryptophan is the last eluted peak, with a retention time of approximately 27 min. For the analysis of tyrosine and 3NT, omit the methanol from the solvent. The elution of 3NT occurs toward the end, with a retention time of approximately 25 min. The amino acids used as calibration standard are prepared in 5% metaphosphoric acid. Amino acids can be detected with a Model 5600 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +600, +700, +800, and +900 mV. With a signal-to-noise ratio of 4:1, the lower limit for electrochemical detection is 300 fmol for 3-nitrotyrosine and 4- and 5-nitrotryptophan and 200 fmol for tyrosine, tryptophan, 5-hydroxytryptophan, and kynurenine. For quantification, inject each sample twice and average the peak areas. Representative HPLC chromatograms are shown Fig. 19.2. The separation of standards (tyrosine, 3NT, tryptophan, 4-and 5-nitrotryptophan) is shown in trace A ( Fig. 19.2). Amino acid analysis of the SCOT carboxy-terminal fragment (trace B, Fig. 19.2) indicates the absence of 3NT and 4- and 5-tryptophan. The amino acid nitrohydroxytryptophan (W OH,N with a retention time of 21 min) occurs exclusively in the full-length protein and the C-terminal fragment. The UV absorption spectrum of nitrohydroxytryptophan, detected with a diode array, is similar to those of nitrated aromatic amino acids, with a characteristic emission peak at 340 to 360 nm. The retention time and UV spectrum of nitrohydroxytryptophan observed during HPLC analysis of the digest of the SCOT protein should be identical to the tryptophan derivative produced in vitro (trace C, Fig. 19.2 ).
What is the calibration standard for amino acids?
The amino acids used as calibration standard are prepared in 5% metaphosphoric acid. Amino acids can be detected with a Model 5600 CoulArray electrochemical detector (ESA, Chelmsford, MA), equipped with a four-channel analytical cell, using potentials of +600, +700, +800, and +900 mV.
Where does propionyl-CoA come from?
Thus, propionyl-CoA is derived from the catabolism of lipids and proteins. In ruminants, propionate is largely derived from bacterial fermentation in the rumen. Propionyl-CoA is converted to succinyl-CoA, which is oxidized or converted to glucose by way of oxaloacetate and pyruvate (gluconeogenesis; Chapter 15).
Succinyl CoA Definition
Succinyl CoA stands for succinyl coenzyme A and is a thioester of coenzyme A and succinic acid.
Overview of Succinyl Coa
The molecular formula of succinyl CoA is C₂₅H₄₀N₇O₁₉P₃S and its molecular structure. Succinyl CoA is produced from methylmalonyl CoA. For this purpose, methylmalonyl-CoA mutase utilizes deoxyadenosyl-B₁₂.
Succinyl CoA in Citric Acid Cycle
Succinyl CoA is one of the main intermediate compounds of the citric acid cycle. α-ketoglutarate dehydrogenase enzyme helps in the synthesis of α-ketoglutarate by the process of decarboxylation. α-ketoglutarate is later converted to succinate.
Role of Succinyl CoA in Heme synthesis
Heme is an organic compound made of 4 pyrrole rings with an iron molecule in between the ring structure. It has 4 carbon and 1 nitrogen atom. Synthesis of heme is initiated with the condensation of succinyl CoA and glycine (amino acid) in mitochondria.
Succinyl Coenzyme A synthetase (SCS)
It is also known as succinyl CoA or succinate thiokinase or succinate CoA ligase. The enzyme carries out a reversible reaction converting succinyl CoA to succinate. During the reaction, it utilizes inorganic phosphate to produce nucleoside triphosphate such as GTP or ATP in addition to nucleoside diphosphate (GDP or ADP).
What happens if you have a high SCOT level?
People with SCOT deficiency usually have a permanently elevated level of ketones in their blood (persistent ketosis). If the level of ketones gets too high, which can be brought on by infections, fevers, or periods without food (fasting), a ketoacidotic attack can occur.
What happens when you have mutations in OXCT1?
A reduction in the amount of functional enzyme leads to an inability to break down ketones, resulting in decreased energy production and an elevated level of ketones in the blood.
What is a SCOT deficiency?
Succinyl-CoA:3-ketoacid CoA transferase (SCOT) deficiency is an inherited disorder that impairs the body's ability to break down ketones, which are molecules produced in the liver during the breakdown of fats. The signs and symptoms of SCOT deficiency typically appear within the first few years of life.
How long does it take for a scot deficiency to manifest?
The signs and symptoms of SCOT deficiency typically appear within the first few years of life. Affected individuals experience episodes of extreme tiredness (lethargy), appetite loss, vomiting, rapid breathing, and, occasionally, seizures. These episodes, which are called ketoacidotic attacks, sometimes lead to coma. About half of affected individuals have a ketoacidotic attack within the first 4 days of life. Affected individuals have no symptoms of the disorder between ketoacidotic attacks.
How many cases of SCOT deficiency are there?
The prevalence of SCOT deficiency is unknown. More than 20 cases of this condition have been reported in the scientific literature.
How long does it take for a keto attack to happen?
About half of affected individuals have a ketoacidotic attack within the first 4 days of life. Affected individuals have no symptoms of the disorder between ketoacidotic attacks.
What is autosomal recessive gene?
This condition is inherited in an autosomal recessive pattern, which means both copies of the gene in each cell have mutations. The parents of an individual with an autosomal recessive condition each carry one copy of the mutated gene, but they typically do not show signs and symptoms of the condition.
What Other Drugs Interact with Succinylcholine?
If your doctor has directed you to use this medication for your condition, your doctor or pharmacist may already be aware of any possible drug interactions or side effects and may be monitoring you for them. Do not start, stop, or change the dosage of this medicine or any medicine before getting further information from your doctor, healthcare provider, or pharmacist first.
What Are Warnings and Precautions for Succinylcholine?
It occurs soon after administration and requires immediate treatment of hyperkalemia. Prolonged resuscitation may be required.
What is succinylcholine IV?
Succinylcholine is a skeletal muscle relaxant for intravenous (IV) administration indicated as an adjunct to general anesthesia, to facilitate tracheal intubation, and to provide skeletal muscle relaxation during surgery or mechanical ventilation. Succinylcholine is available under the following different brand names: Anectine and suxamethonium.
How to report succinylcholine side effects?
Serious side effects of succinylcholine include: This is not a complete list of side effects and other serious side effects may occur. Call your doctor for information and medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
What happens if you take pseudocholinesterase?
Patients with atypical or deficient pseudocholinesterase will have prolonged paralysis (such as organophosphate/carbamate poisoning, hyperthermia, burn patient, collagen - vascular disease). Additive/synergistic effects if administered with or following an opioid, sedative or anesthetic agent.
Can you take anectine with suxamethonium?
This medication contains succinylcholine. Do not take Anectine or suxamethonium if you are allergic to succinylcholine or any ingredients contained in this drug. This medication contains succinylcholine.
Can succinylcholine be used during pregnancy?
Use succinylcholine with caution during pregnancy if benefits outweigh risks. Animal studies show risk and human studies are not available or neither animal nor human studies were done. It is not known if succinylcholine is excreted in breast milk; its effect on nursing infants is not known.
