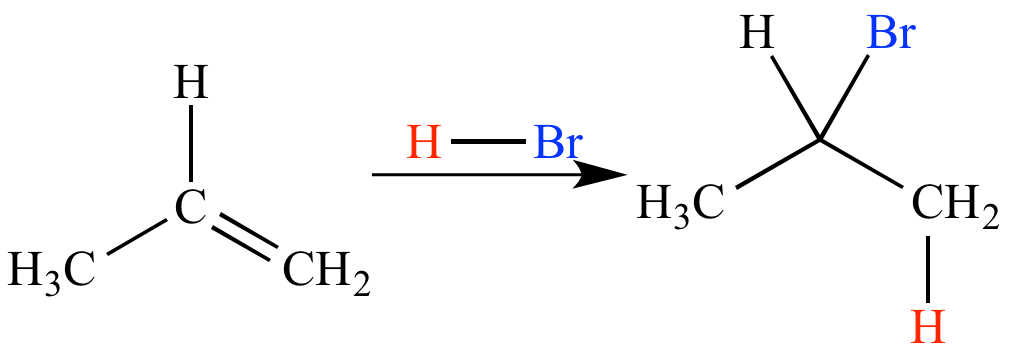
What is syn addition and anti addition reaction?
An addition reaction of an alkene or an alkyne in which the net reaction is addition of two ligands to the multiple-bonded carbon atoms from the same face of the multiple bond is called a syn addition; one in which the net reaction is addition of the two ligands from the opposite faces of the multiple bond is called an anti addition.
What is a syn-addition reaction?
Through this addition reaction, the added atoms/groups can be added to the same side of the double bond (but still across the double bond) which is called a syn-addition, or they could be added to opposite sides, which is called an anti-addition.
Why is Hydroboration-oxidation a syn addition reaction?
This hydroboration-oxidation reaction is a syn addition because this reaction delivers an H and OH to the same face of the alkene. This Diels-Alder reaction is a syn cycloaddition reaction because the two new carbon-carbon sigma bonds are formed on the same face of the diene or dienophile.
What are the two classifications of addition reactions?
For polar addition reactions there are two classifications, namely: For non-polar addition reactions, we have two classifications, namely: An electrophilic addition reaction can be described as an addition reaction in which a reactant with multiple bonds as in a double or triple bond undergoes has its π bond broken and two new σ bonds are formed.
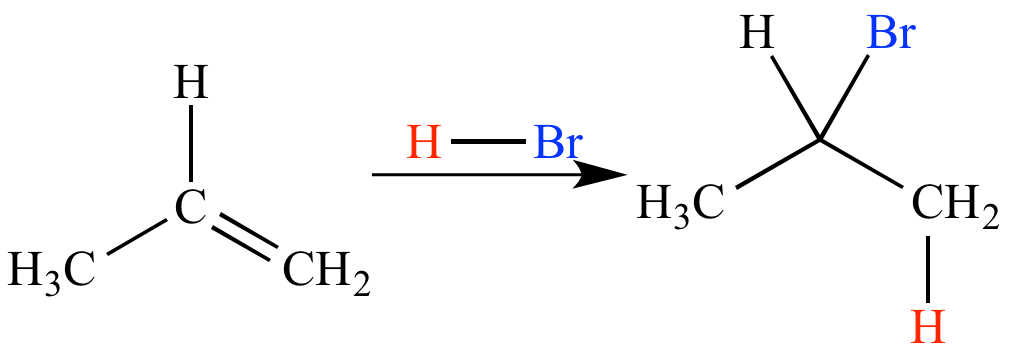
What are syn additions?
An addition reaction in which all new bonds are formed on the same face of the reactant molecule.
What is an anti-addition in chemistry?
An addition reaction in which two substituents are added to opposite sides (or faces) of a double bond or triple bond.
What are syn and anti-elimination?
In syn elimination, the base attacks the β-hydrogen on the same side as the leaving group. In anti-elimination, the base attacks the β-hydrogen on...
What are syn and anti isomers?
The prefixes syn and anti are used to denote geometrical isomerism. Syn: indicates that both H and OH are present on the same side of the double bo...
What do E and Z mean in organic chemistry?
E isomers are alkenes having the substituents with higher priority on the opposite sides of the double bond. Z isomers are alkenes having substitue...
What is syn addition vs anti addition?
Syn addition is where the groups/atoms are added across the alkene in the same orientation or side, while anti addition is where the atoms/groups a...
What reagents give syn and anti addition?
Adding hydrogen to an alkene give a syn addition, adding a halogen gas like Br2 to an alkene give anti addition, and adding H-X to an alkene can gi...
How do you create a syn addition?
Generally, a syn addition happens in situations like adding hydrogen to an alkene, when the intermediate is not able to rotate the addition site, a...
What is syn and anti-addition?
Addition reactions of alkenes will have specific stereochemistry in the finished product. When two atoms are added to the same side, it's a syn relationship ; when two atoms are added to opposite sides it's an anti relationship.
What is an anti addition reaction?
Anti-Addition Reaction. Anti-addition occurs when a halogen (X 2, such as Cl 2 or Br 2 ), is added to an alkene. Each halogen is added to opposite sides of the molecule, like you can see on your screen now: When a halogen such as bromine is added to an alkene, only the anti product forms.
What is an oversimplified addition reaction?
In addition reactions two atoms are added to a double or triple bond, reducing it to a single or double bond. The two atoms can either be added to the same side or to opposite sides of the molecule.
What is it called when two atoms are added to the same side?
When the two atoms are added to the same side, this is called a syn relationship, while the two atoms added to opposite sides is called an anti relationship. These reactions can be stereospecific or create enantiomers, which are two molecules with ...
Why is syn product formed?
Only the syn product is formed. The reason has to do with the mechanism for adding hydrogen to an alkene. We're actually first plating hydrogens onto a thin metal sheet (the Pd-C). This plating keeps the hydrogen atoms from freely rotating around the molecule, so they must be added to the same side of the molecule.
When hydrogen and halide are added to the double bond, the syn and anti forms are formed in close to equal?
When the hydrogen and halide are added to the double bond, the syn and anti forms are formed in close to equal amounts. So, the actual reaction, with stereochemistry identified, would look like this: Syn and anti are produced. As you can see, there are really two products formed in equal proportions.
Can hydrogen be added to an alkene?
Note: this is an extremely oversimplified version of the mechanism for adding hydrogen to an alkene; the entire mechanism is complicated and beyond the scope of this lesson. Just remember that the hydrogen atoms are unable to rotate and need to be added to the same side since they're bound to a metal plate, thus forming the syn product.
What is the name of the reaction in which the net reaction is addition of two ligands to the multiple?
Syn Addition. An addition reaction of an alkene or an alkyne in which the net reaction is addition of two ligands to the multiple-bonded carbon atoms from the same face of the multiple bond is called a syn addition; one in which the net reaction is addition of the two ligands from the opposite faces of the multiple bond is called an anti addition.
What is the net reaction of an alkene?
An addition reaction of an alkene or an alkyne in which the net reaction is addition of two ligands to the multiple-bonded carbon atoms from the same face of the multiple bond is called a syn addition; one in which the net reaction is addition of the two ligands from the opposite faces of the multiple bond is called an anti addition.
What is syn addition?
This article will use cycloalkenes as examples. Syn addition is the addition of two substituents to the same side (or face) of a double bond or triple bond, resulting in a decrease in bond order ...
What happens after addition to a straight chain alkene?
After addition to a straight-chain alkene such as C 2 H 4, the resulting alkane will rapidly and freely rotate around its single sigma bond under normal conditions (i.e. room temperature ).
Can substituents be added to the same side of a double?
Thus whether substituents are added to the same side ( syn) or opposite sides (anti) of a double can usually be ignored due to free rotation. However, if chirality or the specific absolute orientation of the substituents needs to be taken into account, knowing the type of addition is significant.
What is an Addition Reaction?
In the simplest of terms of organic chemistry, we can say that an addition reaction is a chemical reaction wherein two or more reactants come together to form a larger single product.
What is an electrophilic addition reaction?
An electrophilic addition reaction can be described as an addition reaction in which a reactant with multiple bonds as in a double or triple bond undergoes has its π bond broken and two new σ bonds are formed.
What is halogen addition?
A halogen addition reaction is a simple organic reaction in which a halogen molecule is added to an alkene functional group’s carbon–carbon double bond. This reaction is a combination of halogenation and electrophilic addition.
What is nucleophilic addition?
Nucleophilic addition: A nucleophilic addition reaction is an addition reaction where a chemical compound with an electron-deficient or electrophilic double or triple bond, a π bond, reacts with a nucleophile which is an electron-rich reactant with the disappearance of the double bond and creation of two new single, or σ, bonds.
What happens to alkenes in addition?
In the presence of a catalyst, alkenes undergo an addition reaction with water to create alcohol. Hydration is the name for this sort of addition reaction. Water is directly injected into the carbon–carbon double bond.
What happens when an atom is added to a combination with a double or triple bond?
When an atom is added to a combination with a double or triple bond, an addition reaction occurs. Addition reactions are linked to unsaturated molecules. These are hydrocarbons with two or three double or triple bonds. After an addition reaction is completed, there are no reactant residues left.