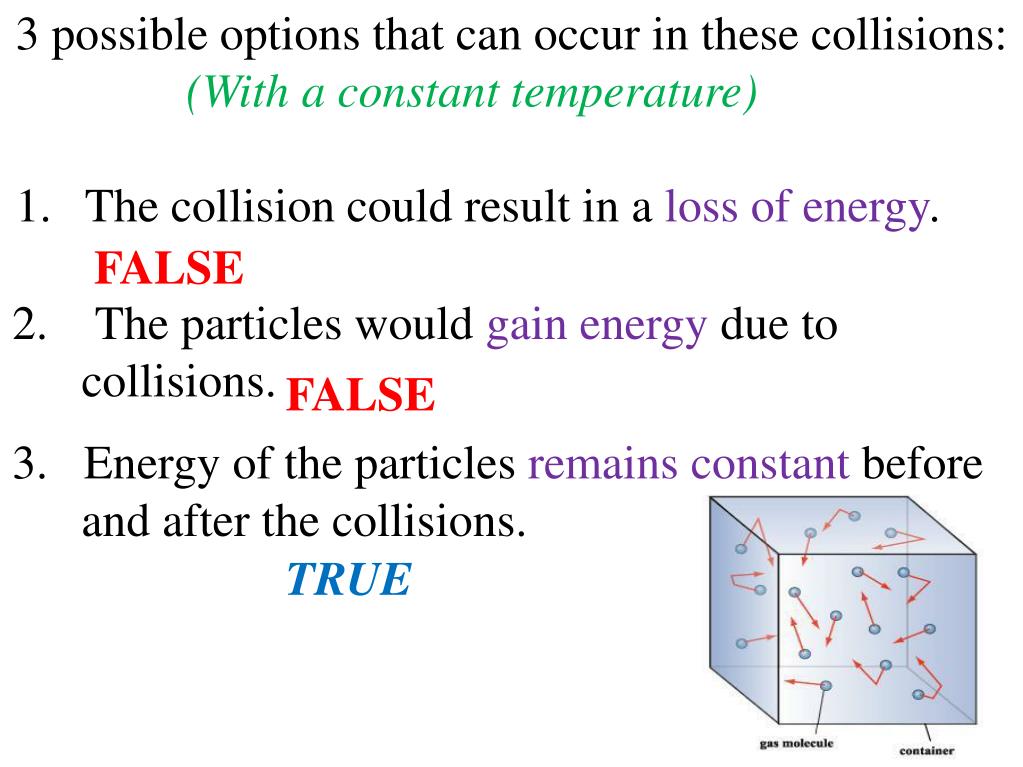
As stated in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, some of the absorbed energy is stored within the particles, while some of the energy increases the speeds at which the particles are moving.
What is the molecule theory?
What happens to the gas molecules when the temperature is increased?
Why do molecules have varying speeds?
How fast does oxygen move?
What happens to the temperature of a gas when the volume of the gas increases?
What is the test of KMT?
What are the learning objectives of gas laws?
See 2 more

What is temperature in kinetic theory?
As stated in the kinetic-molecular theory, the temperature of a substance is related to the average kinetic energy of the particles of that substance. When a substance is heated, some of the absorbed energy is stored within the particles, while some of the energy increases the motion of the particles.
What is the molecular theory of temperature?
It is based on the dependence of temperature on the kinetic energy of the rapidly moving particles of a substance. According to the theory, energy and momentum are conserved in all collisions between particles, and the average behavior of the particles can be deduced by statistical analysis.
What is temperature with kinetic energy?
In chemistry, we define the temperature of a substance as the average kinetic energy of all the atoms or molecules of that substance. Not all of the particles of a substance have the same kinetic energy.
How does temperature apply to kinetic-molecular theory?
Applying Kinetic Theory to Gas Laws According to kinetic molecular theory, an increase in temperature will increase the average kinetic energy of the molecules.
What does kinetic-molecular theory explain?
The kinetic-molecular theory explains the states of matter, and is based on the idea that matter is composed of tiny particles that are always in motion. This theory helps explain observable properties and behaviors of solids, liquids, and gases.
Why does temperature depend on kinetic energy?
As the temperature increases the movement of the particle become high. This lead to increase in kinetic energy. With the increases in temperature the motion of particle is higher in gas molecules than in solid molecules.
What you mean by temperature?
Temperature is the measure of hotness or coldness expressed in terms of any of several scales, including Fahrenheit and Celsius. Temperature indicates the direction in which heat energy will spontaneously flow—i.e., from a hotter body (one at a higher temperature) to a colder body (one at a lower temperature).
What is a simple definition of temperature?
Temperature is the degree of hotness or coldness of an object.
Who mean kinetic temperature?
Mean kinetic temperature (MKT) is a simplified way of expressing the overall effect of temperature fluctuations during storage or transit of perishable goods. The MKT is widely used in the pharmaceutical industry.
What is heat of temperature on the basis of molecular theory?
Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance.
What is the relationship of temperature to the kinetic energy of particles?
The temperature of a substance is directly proportional to the average kinetic energy of the substance particles. Because the mass of these particles is constant, the particles must move faster as the temperature rises.
When temperature is increased in a kinetic energy of molecules?
As the temperature increases, the average kinetic energy of molecules increases. From Gay-Lussac's law, at constant volume, as the temperature is increased, pressure increases.
What is heat of temperature on the basis of molecular theory?
Heat is the total energy of the motion of the molecules of a substance, whereas temperature refers to the measure of the average energy of the motions of the molecules in the substance.
What is an example of kinetic-molecular theory?
What are some examples of kinetic molecular theory? Brownian Motion—the random movement of particulate matter caused by collisions with "air" molecules, and Boyle's, Charles', and Gay- Lussac's Laws—are examples of kinetic theory. This theory also emphasizes how temperature influences the states of matter.
How do molecules move in different temperatures?
With an increase in temperature, the particles gain kinetic energy and move faster. The actual average speed of the particles depends on their mass as well as the temperature – heavier particles move more slowly than lighter ones at the same temperature.
When the temperature increases the molecules move?
In the kinetic theory of gasses, increasing the temperature of a gas increases in average kinetic energy of the molecules, causing increased motion. This increased motion increases the outward pressure of the gas, an expected result from the ideal gas equation PV=NkT.
What happens to the gas molecules when the temperature is increased?
Amontons’s law. If the temperature is increased, the average speed and kinetic energy of the gas molecules increase. If the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with the walls of the container, therefore increasing the pressure (Figure 9.7.1).
What happens to the temperature of a gas when the volume of the gas increases?
Charles’s law. If the temperature of a gas is increased, a constant pressure may be maintained only if the volume occupied by the gas increases. This will result in greater average distances traveled by the molecules to reach the container walls, as well as increased wall surface area. These conditions will decrease the both the frequency of molecule-wall collisions and the number of collisions per unit area, the combined effects of which outweigh those of increased collision forces due to the greater kinetic energy at the higher temperature. The net result is a decrease in gas pressure.
What is KMT gas?
The kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter . This theory is based on the following five postulates described here. (Note: The term “molecule” will be used to refer to the individual chemical species that compose the gas, although some gases are composed of atomic species, for example, the noble gases.)
How fast does oxygen move?
Figure 9.7.2 The molecular speed distribution for oxygen gas at 300 K is shown here. Very few molecules move at either very low or very high speeds. The number of molecules with intermediate speeds increases rapidly up to a maximum, which is the most probable speed, then drops off rapidly. Note that the most probable speed, νp, is a little less than 400 m/s, while the root mean square speed, urms, is closer to 500 m/s.
Do gas molecules have elastic forces?
Gas molecules exert no attractive or repulsive forces on each other or the container walls; therefore, their collisions are elastic (do not involve a loss of energy).
What is the kinetic energy of a gas?
Some particles will have a kinetic energy (and therefore speed, which we will discuss later) that is close to this average value. However, most particles will have a kinetic energy significantly greater or significantly less than this average value. The energy of particle motion is called kinetic energy (KE). The value of this energy averaged over all of the gas molecules in the sample is labeled as KE. For any gas, the temperature (T) and amount of gas ( n, moles) determines the sample’s kinetic energy: KE
Why do molecules have varying molecular speed?
In a gas sample, individual molecules have widely varying speeds. However, because of the large number of molecules and large number of collisions involved , the molecular speed distribution and average speed are constant at a given temperature for that gas sample.
How do gas molecules travel?
Gaseous molecules travel at tremendous speeds (hundreds of meters per second). In general, we know that when a gas sample is introduced to one part of a closed container, its molecules very quickly disperse throughout the container. This process by which molecules disperse in space in response to differences in concentration is called diffusion (shown in Figure 5 ). In a closed environment, diffusion will ultimately result in equal concentrations of gas molecules throughout, as depicted in Figure 5. The gaseous atoms and molecules continue to move, but since their concentrations are the same in both bulbs, the rates of transfer between the bulbs are equal (no net transfer of molecules occurs).
What happens to the pressure of a gas when the temperature is increased?
If the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with the walls of the container, therefore increasing the pressure.
How does the distribution of gas speed change?
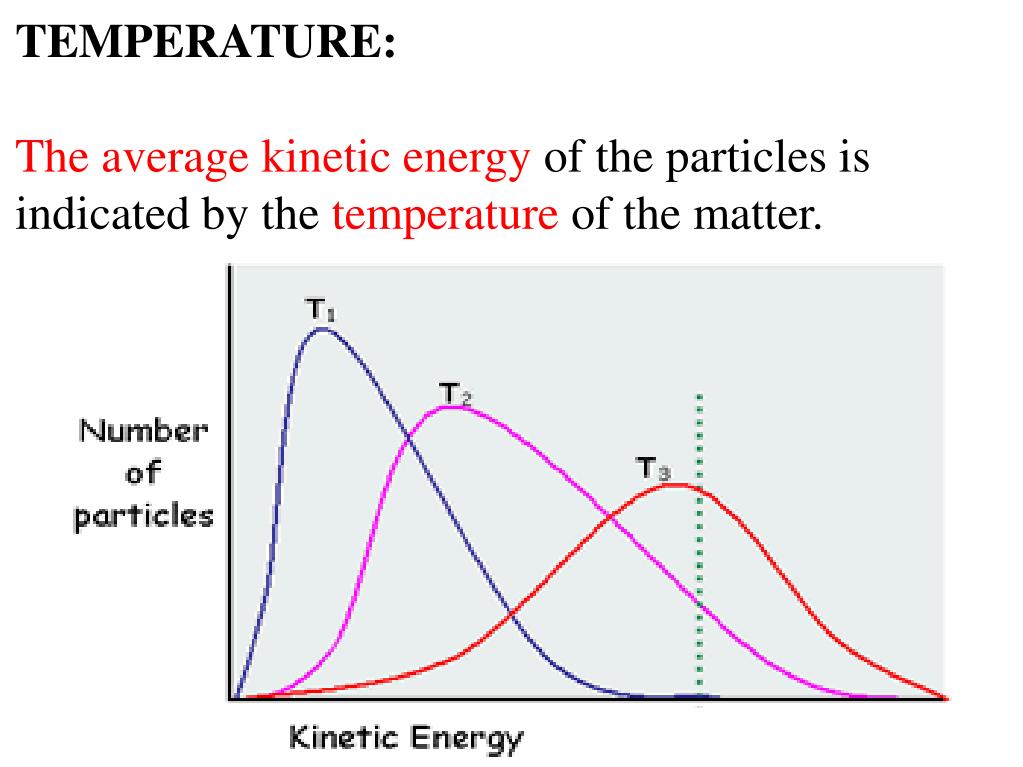
Next, we will consider the distribution of gas speeds in two types of scenarios: either one type of gas at different temperatures or different gases at the same temperature. If the temperature of a gas increases, its KE increases, and more molecules have higher speeds and fewer molecules have lower speeds. Thus, the distribution shifts toward higher speeds overall; that is, to the right. If temperature decreases, KE decreases, and more molecules have lower speeds and fewer molecules have higher speeds. Thus, the distribution shifts toward lower speeds overall; that is, to the left. Notice also that the curve flattens and spreads out as temperature increases. This behavior is illustrated for nitrogen gas in Figure 3.
What is a KMT?
Kinetic molecular theory (KMT) is a simple microscopic model that effectively explains the gas laws described in previous section of this module. This theory is based on the following postulates, which you have seen before in our definition of an ideal gas:
Do gas laws explain the behavior of ideal gases?
Although the gas laws describe relationships that have been verified by many experiments, they do not tell us why gases follow these relationships. This section explores the behavior of ideal gases along with the kinetic molecular theory (KMT) of gases. The kinetic molecular theory will explain these relationships as well as the behavior ...
What are the basic postulates of kinetic molecular theory?
The basic postulates of kinetic molecular theory are as follows: (1) the size of a particle is negligibly small, (2) the average kinetic energy of a particle is proportional to the temperature in kelvins, and (3) the collision of one particle with another is completely elastic. Pressure is defined as force divided by area. According to kinetic molecular theory, a gas is a collection of particles in constant motion. The motion results in collisions between the particles and the surfaces around them. As each particle collides with a surface, it exerts a force upon that surface. The result of many particles in a gas sample exerting forces on the surfaces around them is constant pressure.
What is the process by which gas molecules spread out in response to concentration gradient?
The process by which gas molecules spread out in response to concentration gradient is called diffusion. Effusion is the process by which a gas escapes from a container into a vacuum through a small hole. The rate of effusion is inversely proportional to the sq root of the molar mass of the gas.
How do gases behave?
Gases behave ideally when both of the following are true: (1) The volume of gas particles is small compared to the space between them. (2) The forces between the gas particles are not significant. At high pressures, the number of molecules increases, so the volume of the gas particles is much greater; and because the spacing btwn the particles is much smaller, the interactions become more significant. At low temps, the molecules are not moving as fast as at high temps, so that when they collide, they have a greater opportunity to interact.
Is avg KE proportional to temp?
Postulate 2 of KMT states that the avg KE is proportional to the temp in kelvin. The root mean sq velo of a collection of gas particles is inversely proportional to the square root of the molar mass of particles in kg/mol
What is the molecule theory?
(Note: The term “molecule” will be used to refer to the individual chemical species that compose the gas, although some gases are composed of atomic species, for example, the noble gases.)
What happens to the gas molecules when the temperature is increased?
Amontons’s law. If the temperature is increased, the average speed and kinetic energy of the gas molecules increase. If the volume is held constant, the increased speed of the gas molecules results in more frequent and more forceful collisions with the walls of the container, therefore increasing the pressure ( [link] ).
Why do molecules have varying speeds?
In a gas sample, individual molecules have widely varying speeds; however, because of the vast number of molecules and collisions involved, the molecular speed distribution and average speed are constant.
How fast does oxygen move?
The molecular speed distribution for oxygen gas at 300 K is shown here. Very few molecules move at either very low or very high speeds. The number of molecules with intermediate speeds increases rapidly up to a maximum, which is the most probable speed, then drops off rapidly. Note that the most probable speed, ν p, is a little less than 400 m/s, while the root mean square speed, urms, is closer to 500 m/s.
What happens to the temperature of a gas when the volume of the gas increases?
Charles’s law. If the temperature of a gas is increased, a constant pressure may be maintained only if the volume occupied by the gas increases. This will result in greater average distances traveled by the molecules to reach the container walls, as well as increased wall surface area.
What is the test of KMT?
The test of the KMT and its postulates is its ability to explain and describe the behavior of a gas. The various gas laws can be derived from the assumptions of the KMT, which have led chemists to believe that the assumptions of the theory accurately represent the properties of gas molecules.
What are the learning objectives of gas laws?
The gas laws that we have seen to this point, as well as the ideal gas equation, are empirical, that is, they have been derived from experimental observations. The mathematical forms of these laws closely describe the macroscopic behavior of most gases at pressures less than about 1 or 2 atm.