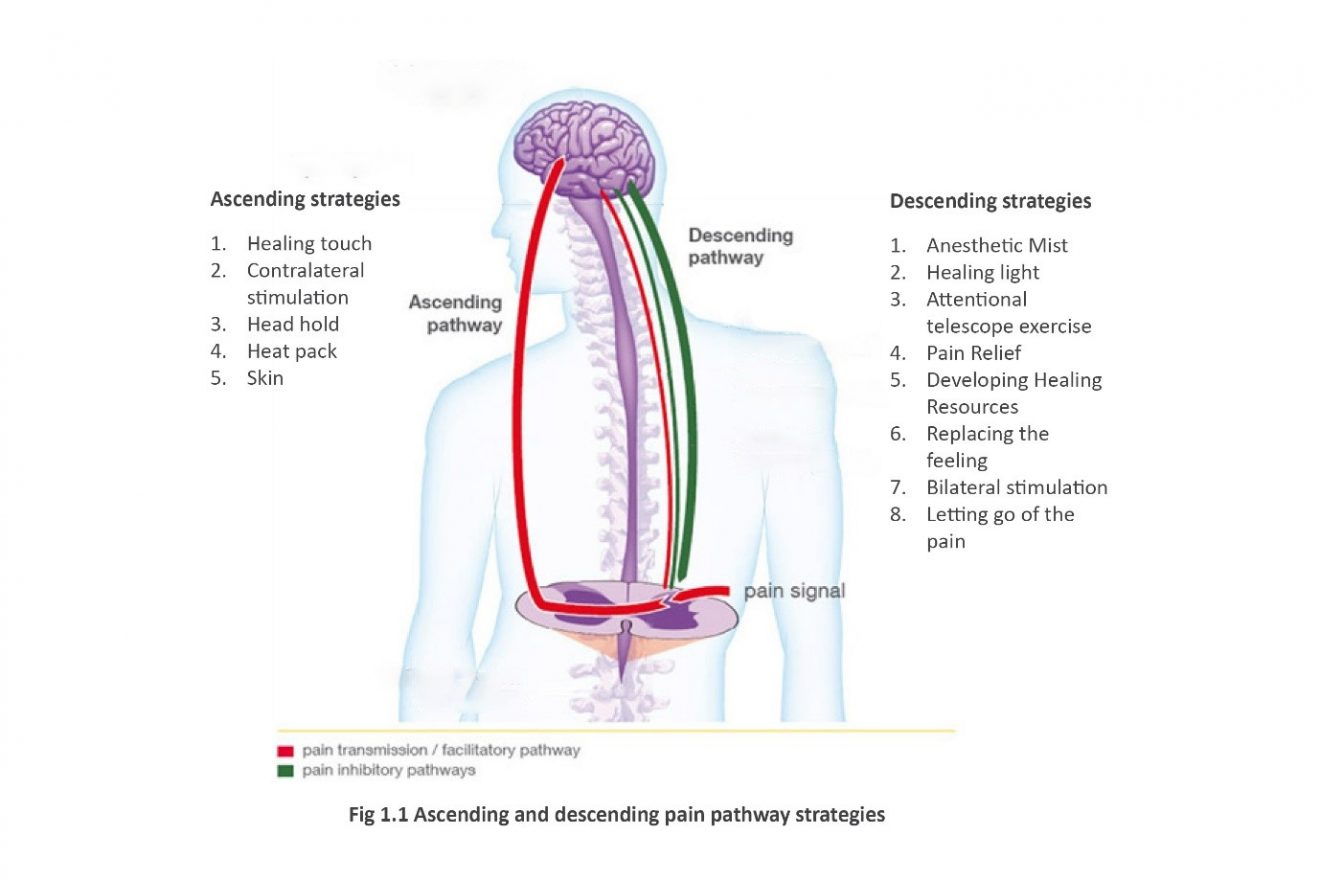
What is the pathophysiology of ascending pain transmission?
Figure 6. Diagram illustrating the route of the ascending transmission pathway. Ascending pain pathway: All opioids, regardless of the initial receptor they bind to, utilize a similar pathway in order to inhibit pain signals. The process begins when pain sensory neurons, called nociceptors, detect a potentially damaging stimulus.
What is the difference between ascending and descending pathway?
Ascending pathway: A nerve pathway that goes upward from the spinal cord toward the brain carrying sensory information from the body to the brain. In contrast, descending pathways are nerve pathways that go down the spinal cord and allow the brain to control movement of the body below the head.
What is the descending pain modulation pathway?
Descending pain pathway: Once the signal from the ascending pathway reaches the somatosensory cortex, it triggers the descending pain modulation pathway. The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition.
How does the pain pathway work in the spinal cord?
Nociceptive primary afferent neurons receive information via free nerve endings at their peripheral terminals and pass that information to second order neurons in the dorsal horn of the spinal cord. This first synapse in the pain pathway is one of the most targeted sites for analgesic drugs.
What is the descending pathway of pain?
The descending pain pathway is a critical modulator of nociception and plays an important role in mediating endogenous and exogenous opioid-induced analgesia. Because of this, it is highly implicated in allostatic cellular and molecular changes following repeated opioid use that lead to the development of tolerance.
What are the 4 stages of pain?
The neurophysiologic underpinnings of pain can be divided into four stages: transduction, transmission, pain modulation, and perception.
Where does the descending pain pathway originate?
EA may block pain by activating the descending pain inhibitory system, which originates in the brainstem and terminates at the spinal cord.
What are the two pain pathways that signal tissue damage?
Substance P (SP) and calcitonin gene-related peptide (CGRP) are released by injury. Inflammation of tissue damage also results in SP and CGRP release, which excites nociceptors.
What are the 3 types of pain?
There are 3 widely accepted pain types relevant for musculoskeletal pain: Nociceptive pain (including nociceptive inflammatory pain) Neuropathic pain. Nociplastic pain.
Which of the following is considered an ascending tract?
Ascending tracts are sensory pathways that begin at the spinal cord and stretch all the way up to the cerebral cortex. There are three types of ascending tracts, dorsal column-medial lemniscus system, spinothalamic (or anterolateral) system, and spinocerebellar system.
What are the descending pathways?
Descending pathways are groups of myelinated nerve fibers that carry motor information from the brain or brainstem to effector's muscles, via the spinal cord. They can be functionally divided into two groups: Pyramidal (voluntary) and extrapyramidal (involuntary) tracts.
What is descending inhibitory pathway?
Descending pain pathway The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition. Descending pain control pathways can be both facilitatory as well as inhibitory. Inhibitory pathways suppresses pain perception.
What are the 4 processes of nociception?
Nociception involves the 4 processes of transduction, transmission, perception, and modulation.
How do you classify pain?
Pain is most often classified by the kind of damage that causes it. The two main categories are pain caused by tissue damage, also called nociceptive pain, and pain caused by nerve damage, also called neuropathic pain. A third category is psychogenic pain, which is pain that is affected by psychological factors.
What are the three mechanisms of pain?
Thus, internationally pain has been classified into three major classes—nociceptive pain, neuropathic pain and inflammatory pain [1]. Primarily, both the CNS and PNS are involved in the mechanism and pathways of all variations of pain perception.
What is the pain pathway called?
These fibers, together with axons from second-order lamina I neurons, form the spinothalamic tract, the major ascending pathway for information about pain and temperature.
What is the descending pain pathway?
Descending pain pathway: Once the signal from the ascending pathway reaches the somatosensory cortex, it trigger s the descending pain modulation pathway. The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition. It begins in the periaqueductal gray (PAG), a region of the midbrain that process nociceptive information and relays it to the rostral ventral medulla (RVM) (11). These neurons in the RVM then send a signal down the spinal cord to release endogenous opioids at neuronal synapses at multiple points in the peripheral nervous system to prevent these pain signaling neurons from sending action potentials. Additionally, these endogenous opioids are released in parts of the dorsal horn of the spinal cord to further block ascending pain transmission signals. For more information about the specific mechanism of neuronal inhibition, visit the section of general opioid signaling. Pain is helpful since it alerts the brain to tissue damage and provides useful information about its type and location. However, when this signal starts to impede our own ability to survive and function it must be reduced, hence the need for the descending pathway and endogenous opioids.
What is the pathway of pain?
Ascending pain pathway: All opioids, regardless of the initial receptor they bind to, utilize a similar pathway in order to inhibit pain signals. The process begins when pain sensory neurons, called nociceptors, detect a potentially damaging stimulus.
What is the goal of the periaqueductal gray pathway?
The goal of this pathway is to allow the organism to function enough to respond to the pain source by reducing the pain signal through neuronal inhibition. It begins in the periaqueductal gray (PAG), a region of the midbrain that process nociceptive information and relays it to the rostral ventral medulla (RVM) (11).
Why is pain important for neuronal inhibition?
Pain is helpful since it alerts the brain to tissue damage and provides useful information about its type and location.
Why split the original pain transmission diagram?
I split the original pain transmission diagram up to address the earlier comment of it being too information rich to be at the start of the page as well prevent the end of the section from being to text-heavy. Thanks for the feedback!
Which neurotransmitter depolarizes the cell and promotes inflammation and pain?
These neurons send this signal as an action potential to other neurons using the excitatory neurotransmitters glutamate, which depolarizes the cell, and substance p, which promotes inflammation and pain (11).
Where does the spinal cord send sensory signals?
Once the signal reaches the base of dorsal horn, a column in the spinal cord that relays sensory information, it is then sent up though a network of neurons called the spinothalamic tract that delivers sensory signals from the spinal cord to the somatosensory cortex which perceives pain (12). This process, from signal detection ...
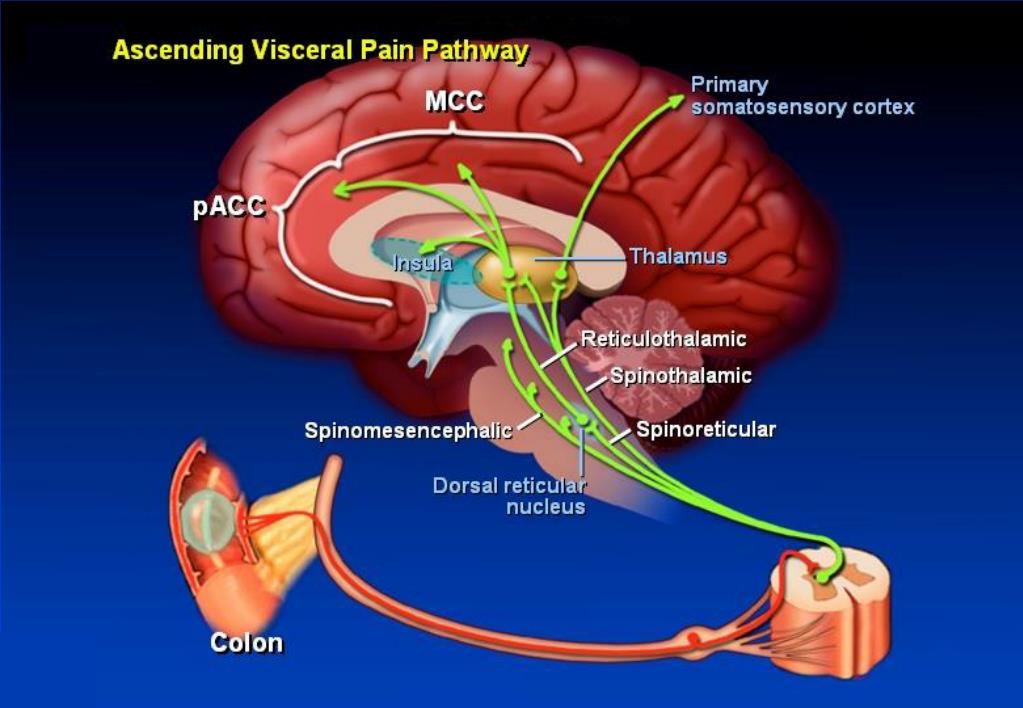
Where does the ascending pain pathway originate?
1 ). In addition, the descending pain pathway originates in higher cortical regions and in the amygdala and hypothalamus, and projects (via the periaqueductal gray (PAG)) to the lower brain stem and spinal cord. Descending control of pain can be either inhibitory or facilitatory depending on the precise circuitry and receptors that are engaged ( Millan, 2002; Ossipov, Morimura, & Porreca, 2014; Suzuki & Dickenson, 2005; Suzuki, Rygh, & Dickenson, 2004 ). The endocannabinoid system is expressed throughout the ascending and descending pain pathways at peripheral, spinal, and supraspinal sites ( Fig. 1 ). CB 1 receptors are located on peripheral endings and central terminals of primary afferent neurons ( Hohmann, Briley, & Herkenham, 1999; Hohmann & Herkenham, 1998, 1999a ). CB 1 receptors are also found in the dorsal root ganglion (DRG) and in the superficial laminae of the spinal cord ( Farquhar-Smith et al., 2000; Glass, Dragunow, & Faull, 1997; Herkenham et al., 1991; Hohmann & Herkenham, 1999b; Ross et al., 2001; Sanudo-Pena, Strangman, Mackie, Walker, & Tsou, 1999 ). Ahluwalia, Urban, Bevan, Capogna, and Nagy (2002) reported that 80% of CB 1 R-expressing neurons either contained calcitonin gene-related peptide (CGRP), a marker for peptidergic neurons, or bound IB4, a marker for an unmyelinated neurons which express glycoproteins ( Ahluwalia et al., 2002 ), suggesting a functional role for CB 1 R on peripheral nerve terminals. However, there is also evidence that CB 1 R mRNA is expressed predominantly in medium- and large-sized DRG neurons, with lower levels in DRG neurons expressing substance P or CGRP mRNA ( Hohmann & Herkenham, 1999b ). In addition to its peripheral and spinal localization, CB 1 R is also located in all of the major brain regions involved in pain processing and modulation. Receptor autoradiography and immunohistochemistry studies have demonstrated the presence of CB 1 R in the cortex, amygdala, hypothalamus, thalamus, PAG, parabrachial nucleus, and in brain stem regions including the rostral ventromedial medulla (RVM) ( Glass et al., 1997; Herkenham et al., 1991, 1990; Mailleux, Parmentier, & Vanderhaeghen, 1992; Thomas, Wei, & Martin, 1992; Tsou, Brown, Sanudo-Pena, Mackie, & Walker, 1998 ). CB 1 R localization is predominantly presynaptic, and its direct activation by synthetic agonists, or by endocannabinoids that signal retrogradely, inhibits the release of neurotransmitters including GABA and glutamate ( Rea, Roche, & Finn, 2007 ).
What are the three main components of the pain pathway?
Mechanisms of pain processing. The ‘pain pathway’ can be split into three principal components: 1. Peripheral tissue nociceptors detect the stimulus and transmit the nociceptive signal via primary afferent nerve fibers to the spinal cord or cranial nerve nuclei. 2.
Where is top down sensitization found?
Top-down sensitization due to stress is not limited to the supraspinal sites of the pain matrix. CORT receptors are expressed throughout the body and are also found on neurons in the periphery. When peripheral neurons interact with circulating CORT, during stress, they will upregulate their expression of pro-nociceptive receptors. These molecular changes underlie the enhancement of visceral pain after acute or chronic stress ( Hong et al., 2011; Luo et al., 2020 ).
What happens after further processing at supraspinal sites?
After further processing at supraspinal sites, the signal induces the conscious perception of pain.
What is the best form of pain therapy?
The optimum form of pain therapy is continuous pre-emptive analgesia, continuously preventing the establishment of sensitization. The administration of opioids or local anesthetic drugs blocks central sensitization and nonsteroidal anti-inflammatory drugs (NSAIDs) reduce the severity of the peripheral inflammatory response. The combined use of an opioid and an NSAID is more effective than using either drug alone. Local anesthetics (analgesics) can produce complete pain relief by blocking all sensory input from the affected area.
Where are the endocannabinoids located?
Thus, the endocannabinoids, N -acylethanolamines, and their metabolizing enzymes are localized in peripheral tissues innervated by primary afferent nociceptive neurons ( Calignano, La Rana, Giuffrida, & Piomelli, 1998; Felder et al., 1996 ), spinal cord ( Di Marzo et al., 2000; Egertova, Giang, Cravatt, & Elphick, 1998; Tsou, Nogueron, et al., 1998 ), and brain ( Devane et al., 1992; Egertova et al., 1998; Hanus et al., 2001; Huang et al., 2002; Porter et al., 2002; Stella, Schweitzer, & Piomelli, 1997; Tsou, Nogueron, et al., 1998) tissues, including regions important in pain. Elegant in vivo microdialysis experiments demonstrated that intraplantar injection of the chemical irritant formalin evokes the release of AEA in the midbrain PAG ( Walker, Huang, Strangman, Tsou, & Sanudo-Pena, 1999 ). The endocannabinoids and N -acylethanolamines also have affinity for, and activity at, a number of non-CB 1 /non-CB 2 receptors, including TRPV1, GPR55 (putative CB 3 receptor), and the peroxisome proliferator-activated receptors (PPARs) ( Alexander & Kendall, 2007; Wiley & Martin, 2002 ), all of which are also expressed throughout the pain pathways and likely play important roles in endocannabinoid-mediated regulation of pain. The remainder of this review will focus on functional in vivo studies of cannabinoids and the endocannabinoid system in models of acute, inflammatory, and neuropathic pain with a focus on supraspinal, spinal, and peripheral sites and mechanisms of action.
What is multimodal analgesia?
Multi-modal analgesia uses different classes of analgesic drugs, which act at different sites in the pain pathway, in combination. The side effects of different drug classes are usually different, so that, with the exception of NSAIDs and steroids, combining drug classes will also not lead to exacerbation of drug side effects.
Where is appendicitis pain located?from oh-mygut.com
with appendicular pain: The pain is sharper and stabbing. Initially (in the first one or two days) the pain is located around the belly button. The pain gradually gets worse and moves to the lower right abdomen.
What does it mean when you feel a stabbing pain in your abdomen?from oh-mygut.com
Severe stabbing pain at the lower right abdomen: may suggest appendicitis (inflammation of your appendix).#N#However, this can also occur with serious colon conditions such as mesenteric vascular occlusion or perforated colon.
What is the name of the artery that the ascending colon gets its blood supply from?from oh-mygut.com
Mesenteric vascular occlusion: the ascending colon gets its blood supply from an artery called “superior mesenteric artery ”. Obstruction of blood supply to the ascending colon can result in an emergency medical condition with severe abdominal pain (right-sided or generalized abdominal pain).
What is the concept of peripheral sensitization?from seekhealthz.com
The concept of peripheral and central sensitization is important for an understanding of widespread chronic pain conditions such as FM. With continuous and prolonged noxious stimulation (e.g., whiplash injury), peripheral polymodal C fibers and nearby silent nociceptive neurons that were previously unresponsive to stimulation now become responsive . The nociceptors begin to initiate signals spontaneously so that non-noxious stimuli are now perceived as noxious due to lowered pain threshold (peripheral sensitization). The result of peripheral sensitization causes a greater and more persistent barrage of nerve impulses to the dorsal root of the spinal cord. Release of substance P by C fibers sensitizes second-order neurons, including WDR neurons, to neurotransmitters, such as glutamate. The enhanced and persistent glutamate effect on second-order neurons can result in physiologic changes in the nerves so they become hyperexcitable. This is called windup. These hyperexcitable second-order neurons transmit excessively to the brain areas described earlier, resulting in expanded receptive fields, increased interconnectivity, and increased blood flow to the stimulated areas. The ability to expand the receptive field is called neuroplasticity and occurs more easily in younger brains, which may explain why painful events early in life are particularly likely to trigger FM. Due to these physiologic changes, central sensitization occurs so that the individual feels pain at a lower threshold and with increased intensity.
Why does my right side of my abdomen hurt?from oh-mygut.com
The most common causes are infections (gastroenteritis), irritable bowel syndrome, and IBD. The ascending colon pain is colicky in nature (waves of contractions followed by relaxation). Other organs and muscles on the right side of your abdomen can cause pain similar to the ascending colon pain. Most commonly: Biliary pain, appendix pain, female ...
What does it mean when your back hurts?from oh-mygut.com
Sudden, intense pain at the right side of your back that may spread to the groin may suggest right renal colic. Severe tendernes s over your upper or lower right abdomen: is not a usual ascending colon symptom. Fever, Severe nausea, vomiting: is not a feature of simple colon spasms.
Where does IBS pain come from?from oh-mygut.com
Irritable bowel syndrome can cause pain anywhere in your abdomen. According to this study, the most common IBS pain locations are: Around the belly button. Upper central part of your abdomen (below the ribs). The left side of your abdomen (descending colon pain).
Which pathways are responsible for psychogenic pain?
The influences of the descending pathways might also be responsible for psychogenic pain (pain perception with no obvious physical cause).
Which direction does pain influence neural pathways?
This indicates that pain-influencing neural pathways must exist from the brain downward.
How does pain affect the nervous system?
Pain signals can set off autonomic nervous system pathways as they pass through the medulla, causing increased heart rate and blood pressure, rapid breathing and sweating. The extent of these reactions depends upon the intensity of pain, and they can be depressed by brain centers in the cortex through various descending pathways.
What happens when you distract yourself from pain?
If you consciously distract yourself, you don't think about the pain and it bothers you less. People given placebos for pain control often report that the pain ceases or diminishes. This indicates that pain-influencing neural pathways must exist from the brain downward. These descending pathways originate in the somatosensory cortex (which relays ...
What neurotransmitters are used to treat pain?
Some of this relief comes from the stimulation of natural pain-relieving opiate neurotransmitters called endorphins, dynorphins and enkephalins. Pain signals can set off autonomic nervous system pathways as they pass through the medulla, causing increased heart rate and blood pressure, rapid breathing and sweating.
How to describe pain?
Physicians and neuroscientists generally classify pain in the following ways: 1 Acute pain is caused by an injury to the body. It warns of potential damage that requires action by the brain, and it can develop slowly or quickly. It can last for a few minutes to six months and goes away when the injury heals. 2 Chronic pain persists long after the trauma has healed (and in some cases, it occurs in the absence of any trauma). Chronic pain does not warn the body to respond, and it usually lasts longer than six months. 3 Cancer (or malignant) pain is associated with malignant tumors. Tumors invade healthy tissues and exert pressure on nerves or blood vessels, producing pain. Cancer pain can also be associated with invasive procedures or treatments. Some physicians classify cancer pain with chronic pain.
Why do women have a higher sensitivity to pain than men?
This could be because of sex-linked genetic traits and hormonal changes that might alter the pain perception system. Psychosocial factors could be at work, too -- men are expected not to show or report their pain.
Which pathway is the ascending pain pathway?
Ascending pain pathway is the pathway with afferent fibres. Lateral spinothalamic tract is the ascending tract which carry pain from pheriphery to central. Free nerve endings at tissue level are triggered by inflammatory mediators (Cytokines such as IL-1b, IL-6 and TNF ,prostaglandins) from immune cells of peripheral tissues after any injury. Aδ afferent fibers,which transmit impulses of fast pain secrete glutamate.The C type fibers, which transmit impulses of slow pain secrete substance P. Glutamate and Sustance p are two neurotrasmiters which help to transmit impluse from nerve endings i.e 1st order neuron to 2nd order neuron. 2nd order neuron run from dorsal horn of spinal cord (sustania gelatinosa) to opposite thalamus . The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for regulation of nociceptive transmission by both local segmental and supraspinal mechanisms. Then 3rd order neuron from thalamus to cortex (primary and secondary somatosensory cortex (S1 and S2 respectively), anterior- and mid-cingulate cortex (ACC and MCC, respectively) and insula).
Which pathway is involved in endogenous pain modulation?
Serotonergic pathway. Serotonin (5-HT) and norepinephrine, are involved in endogenous pain modulation. Norepinephrine and 5-HT can be released via descending pain pathways to modulate nociceptive signaling in the spinal cord.
What is supraspinal pain modulation?
Accumulating evidence supports the important role of supraspinal pain modulation for both analgesia and hyperalgesia. Multiple cortical and subcortical brain and brainstem regions integrate and process sensory, autonomic and emotional information, resulting in activation of the PAG and RVM, with subsequent inhibition or facilitation of pain-related dorsal horn neurons. This top–down modulation is relevant for experimental, as well as clinical pain. These pain modulatory pathways are affected by memories and mood, as well as sociocultural background as different cortical regions like amygdala, hypothalamus are involved in the descending pain modulation pathways.
What is descending pain control?
Supraspinal (or descending) pain control pathways arises from a number of supraspinal sites. Descending pain control pathways can be both facilitatory as well as inhibitory. Facilitatory pathways are the one which enhances pain perception where as inhibitory pathways suppresses pain perception.
What is the balance between inhibition and facilitation?
The balance between inhibition and facilitation is dynamic , and can be altered in different behavioral, emotional, psychological and pathological states. Descending pain control pathways plays a critical role in determining the experience of both acute and chronic pain.
Does PAG increase 5-HT?
Direct stimulation of PAG or RVM does not only increase 5-HT but also norepinephrine concentrations in the cerebrospinal fluid, resulting in pain reductions Although neither PAG nor RVM contain noradrenergic neurons, both regions communicate with norad - renergic brain stem nuclei associated with pain modulation, including the locus coeruleus. These nuclei have noradrenergic projections to the spinal cord, which can inhibit the response of dorsal horn pain transmission neurons.Dorsal horn neuron recordings have shown that activated a2-adrenergic receptors hyperpolarize presynaptic neurons and decrease the release of excitatory neu - rotransmitters from primary afferent terminals, resulting in pain inhibition.
Which horn is the first synapse?
The dorsal horn of the spinal cord is the location of the first synapse in pain pathways, and as such, offers a very powerful target for regulation of nociceptive transmission by both local segmental and supraspinal mechanisms.
What are the steps of the pain pathway?
The 4 Steps of the Pain Pathway: Transduction, Transmission, Modulation, and Perception
Which nerves are responsible for sharp pain?
The primary afferent nerves that contain these nociceptors are either A delta fibers, which are large and myelinated and responsible for acute sharp pain, or C fibers, which are small and unmyelinated and responsible for slow-onset, dull, lingering, or achy pain.
How do inflammatory mediators sensitize the nociceptors?
How do the inflammatory mediators sensitize the nociceptors? By PGE2 binding to specific areas on the nociceptors on A delta and C fibers (creating cyclic AMP from ATP), and by altering voltage-gated sodium channels , the depolarization threshold is lowered, meaning minimal nonpainful stimuli cause a nociceptive action potential to be produced (allodynia) and painful stimuli to trigger more action potentials (hyperalgesia). Furthermore, other receptors, such as transient receptor potential vanilloid 1 (TRPV1), which is sensitive to high temperatures, will be altered to fire at lower temperature levels, thus creating temperature-dependent allodynia. The C fiber nociceptors can be altered to the point that the threshold potential is at resting potential, so action potentials are continuously produced, creating ongoing unrelenting pain during inflammation.
How do gabapentinoids affect neuropathy?
The VGCCs are upregulated within damaged neurons as an adaptive response leading to an exaggerated influx of calcium and neurotransmitter release , with the arrival of an action potential, leading to neuropathic pain, demonstrating the obvious benefit of the administration of gabapentinoids for treating neuropathy. However, their effectiveness for treating acute pain is less obvious. This class of drugs is likely not capable of being the sole modality for treating acute surgical pain, and the effect is known to be present but limited, which is consistent with the above-described mechanism of action and known upregulation with nerve damage, but not acute tissue injury. Other mechanisms involving NMDA receptor blockade, inhibition of Na+ currents, and increased serotonin and norepinephrine activity leading to stimulation of the descending pain modulation pathway have been described (Figures 10 and 11).
How does perception occur?
Perception occurs when the nociceptive signal is received by the involved cortexes within the brain. The individual becomes aware of the insult, and an emotional and motor response is initiated. It has reached consciousness and now moves from nociception to pain.
What is the action potential of the visceral primary afferent?
Since the visceral primary afferents are usually “silent ,” their action potentials are frequently interpreted by the cortexes as signals coming from other “commonly” active primary afferent neurons within the same part of the body. This leads to “referred pain.”.
What is the difference between nociception and pain?
Pain is the unpleasant experience caused by potential or actual tissue damage, and nociception is the transmission of a noxious stimulus to the brain and all the processes in between.
What is the function of pain?
Pain is a complex and generally unpleasant experience that serves the important protective function of alerting us to situations that may threaten our well being. As such, pain is typically associated with noxious stimuli, events that are potentially or actually damaging to tissue. Pain processing begins with specialized sensory neurons called nociceptors that are able to distinguish and preferentially respond to noxious stimuli. Nociceptive primary afferent neurons receive information via free nerve endings at their peripheral terminals and pass that information to second order neurons in the dorsal horn of the spinal cord. This first synapse in the pain pathway is one of the most targeted sites for analgesic drugs. From here, several ascending pathways exist to relay messages related to arousal as well as affective and other aspects of pain. The most prominent ascending pain pathway is the
Which part of the spinal cord is the main site of action for opioids?
The dorsal horn of the spinal cord is main site of action for opioids, where inhibition of VGCCs resulting from G
What enzymes are used to treat pain?
Enzymes that degrade endogenous cannabi- noids are another pharmacological target for pain therapy. One of the most commonly stud- ied is fatty acid amide hydrolase (FAAH), the major degrading enzyme of the endogenous cannabinoid AEA. Normally, AEA is rapidly degraded, limiting its analgesic efficacy. Inhibi- tion of FAAH results in more robust, longer last- ing AEA action and does so without producing the psychomimetic effects elicited by some cannabinoid receptor agonists, making it a par- ticularly attractive target [2]. Since pharmacological FAAH inhibition increas- es endogenous cannabinoid levels, it should not be surprising that activation of CB1 and/or CB2 receptors is the primary mechanism by which this drug class produces its antinociceptive effects. However, FAAH catabolizes fatty acid amides other than AEA, and cannabinoid recep- tor agonists can act at other receptor types. As such, non-cannabinoid mechanisms of action can contribute to the analgesic effects produced by FAAH blockade. For example, both TRPV1 and opioid receptor activation have been shown to play a role in FAAH inhibitor-mediated antinoci- ception in some models [2]. Pharmacological targeting of the cannabinoid system, either through receptor activation or FAAH blockade, is a useful analgesic strategy for a wide variety of pain models. Cannabinoid ago- nists and FAAH inhibitors have shown efficacy in acute models such as tail flick and capsaicin injection, as well as carrageenan and CFA inflam- matory pain models. Translation from animal models to the human condition has been docu- mented for a variety of neuropathic conditions as well as for post-operative pain relief; there- fore, both neuropathic and post-operative pain models would be appropriate for testing novel compounds designed to target the cannabinoid system as well [1].
Where are AARs expressed?
AARs are expressed mostly on the central, pre-synaptic terminals of nociceptors and inhib- it VGCC on these terminals to reduce the release of excitatory neurotransmitters such as gluta- mate and substance P. At the same time, α
