What are facts about aluminum?
Fascinating facts about aluminum! Aluminum is lightweight, strong, corrosion-resistant, infinitely recyclable, and an essential part of our daily life. Comprising a little over 8% of the earth’s crust, aluminum is the most abundant metal on the planet. It is the third most common element after oxygen and silicon.
How many protons neutrons and electrons does aluminum have?
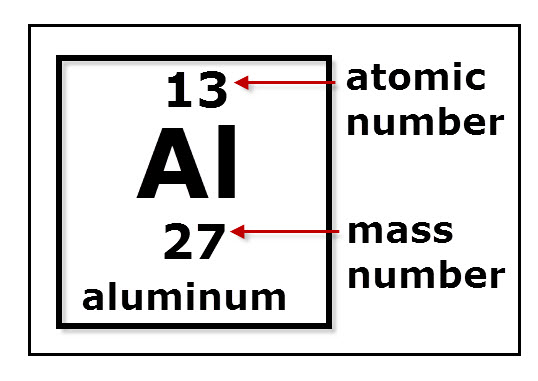
Therefore, for an atom of aluminum, there are 13 electrons inside it. So we’ve demonstrated that for an aluminum atom which has the atomic number of 13 and has a mass number of 27, there are 13 protons, 14 neutrons, and 13 electrons. READ: What does triangle with circle tattoo mean?
What is the molecular structure of aluminum?
The molecular geometry of AlCl3 is trigonal planar because the central atom aluminium is bonded with three chlorine atoms and it contains no lone pair, this means, distortion around the central position will not happen because of no lone pair. Hence, the three bonded atoms (chlorine) are arranged like a triangle around the central atom (aluminium)
What is the Amu of aluminum?
The atomic mass of aluminum is 26.98 amu. Aluminum is one of the lightest elements, making it ideal for construction, transportation and other commercial uses. Aluminum is a very common element, third in abundance only to silicon and oxygen. However, aluminum is too reactive to exist in a pure form naturally.

What is the atomic number of titanium?
Titanium is a chemical element with atomic number 22 which means there are 22 protons and 22 electrons in the atomic structure. The chemical symbol for Titanium is Ti. Titanium is a lustrous transition metal with a silver color, low density, and high strength. Titanium is resistant to corrosion in sea water, aqua regia, and chlorine. Titanium can be used in surface condensers. These condensers use tubes that are usually made of stainless steel, copper alloys, or titanium depending on several selection criteria (such as thermal conductivity or corrosion resistance). Titanium condenser tubes are usually the best technical choice, however titanium is very expensive material.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
Which element has the same electron configuration in the outer electron shell?
Magnesium is a shiny gray solid which bears a close physical resemblance to the other five elements in the second column (group 2, or alkaline earth metals) of the periodic table: all group 2 elements have the same electron configuration in the outer electron shell and a similar crystal structure.
What is the process used to make aluminum?
The principal method used in producing aluminum metal involves three major steps: refining of bauxite by the Bayer process to produce alumina, electrolytic reduction of alumina by the Hall-Heroult process to produce aluminum and casting of aluminumin to ingots.
What is aluminum acetate?
Aluminum Acetate is an astringent. An astrignent is a chemical that tends to shrink or constrict body tissues, usually locally after topical medicinal application. The shrinkage or constriction is through osmotic flow of water (or other fluids) away from the area where the astringent was applied. Astringent medicines cause shrinkage of mucous membranes or exposed tissues and are often used internally to check discharge of blood serum or mucous secretions. This can happen with a sore throat, hemorrhages, diarrhea, or with peptic ulcers. Externally applied astringents, which cause mild coagulation of skin proteins, dry, harden, and protect the skin. Acne sufferers are often advised to use astringents if they have oily skin. Astringents also help heal stretch marks and other scars. Mild astringent solutions are used in the relief of such minor skin irritations as those resulting from superficial cuts, allergies, insect bites, or fungal infections such as athlete's foot.
What is aluminium powder?
Aluminium powder is composed of finely divided particles of aluminium. The grinding may or may not be carried out in the presence of edible vegetable oils and/or food additive quality fatty acids. It is free from admixture with substances other than edible vegetable oils and/or food additive quality fatty acids.
What animals have aluminum dust?
/LABORATORY ANIMALS: Acute Exposure/ ...Inhalation or intratracheal injection... of aluminum dust caused respiratory infections... (rats and rabbits) /showed/ widespread interstitial fibrosis, with hyalinosis, emphysema and hemorrhages... from which often arise bullous emphysema, bronchopneumonia and hemorrhagic pneumonia. These changes were not limited to the pulmonary parenchyma but were present to some degree in the walls of the blood vessels and in the kidneys, and some fibrous thickening of interstitial tissue in the spleen, liver and meninges. / Aluminum dust/
What is the most abundant metal in the world?
Aluminum is the most abundant metal in the earth's crust. It is always found combined with other elements such as oxygen, silicon, and fluorine. Aluminum as the metal is obtained from aluminum -containing minerals. Small amounts of aluminum can be found dissolved in water. Aluminum metal is light in weight and silvery-white in appearance. Aluminum is used for beverage cans, pots and pans, airplanes, siding and roofing, and foil. Aluminum is often mixed with small amounts of other metals to form aluminum alloys, which are stronger and harder. Aluminum compounds have many different uses, for example, as alums in water -treatment and alumina in abrasives and furnace linings. They are also found in consumer products such as antacids, astringents, buffered aspirin, food additives, and antiperspirants.
What temperature does aluminum melt?
Aluminum metal held above melting point of 1220°F (660°C) for ease in handling. Cools and solidifies if released. Contact causes thermal burns. Plastic or rubber may melt or lose strength upon contact. Protective equipment designed for chemical exposure only is not effective against direct contact.
Can aluminum be used as an explosive?
A mixture of aluminum powder and ammonium nitrate can be used as an explosive. A number of explosions in which ammonium nitrate and aluminum are mixed with carbon, hydrocarbons, with or without oxidizing agents, have occurred.
What is the mass number of aluminum?
Mass numbers of typical isotopes of Aluminium are 27.
What is the atomic mass of an atom?
The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element. The atomic mass is carried by the atomic nucleus, which occupies only about 10 -12 of the total volume of the atom or less, but it contains all the positive charge and at least 99.95% of the total mass of the atom. Note that, each element may contain more isotopes, therefore this resulting atomic mass is calculated from naturally-occuring isotopes and their abundance.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
What is the lightest element on the periodic table?
With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Its monatomic form (H) is the most abundant chemical substance in the Universe, constituting roughly 75% of all baryonic mass.
What is the most abundant element in the Earth's crust?
Aluminium is a silvery-white, soft, nonmagnetic, ductile metal in the boron group. By mass, aluminium makes up about 8% of the Earth’s crust; it is the third most abundant element after oxygen and silicon and the most abundant metal in the crust, though it is less common in the mantle below.
What is the atomic radius of an aluminium atom?
The atomic radius of Aluminium atom is 121pm (covalent radius).
What is the radius of a metallic atom?
A metallic radius is one-half the distance between the nuclei of two adjacent atoms in a crystalline structure, when joined to other atoms by metallic bonds.
How many electrons does neon have?
Neon is a chemical element with atomic number 10 which means there are 10 protons and 10 electrons in the atomic structure. The chemical symbol for Neon is Ne.
How many protons does nitrogen have?
Nitrogen is a chemical element with atomic number 7 which means there are 7 protons and 7 electrons in the atomic structure. The chemical symbol for Nitrogen is N.
How many protons does helium have?
Helium is a chemical element with atomic number 2 which means there are 2 protons and 2 electrons in the atomic structure. The chemical symbol for Helium is He.
How is atomic weight determined?
Therefore it is determined by the mass number (number of protons and neutrons).
What is the atomic number density?
Atomic Number Density. The atomic number density (N; atoms/cm 3 ), which is associated with atomic radii, is the number of atoms of a given type per unit volume (V; cm 3) of the material. The atomic number density (N; atoms/cm 3) of a pure material having atomic or molecular weight (M; grams/mol) and the material density (⍴; gram/cm 3) is easily computed from the following equation using Avogadro’s number ( NA = 6.022×1023 atoms or molecules per mole):
What is the mass number of aluminum?
Mass numbers of typical isotopes of Aluminium are 27.
What is the electronegativity of aluminium?
The electronegativity of Aluminium is: χ = 1.61
How many protons and electrons are in hydrogen?
Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure. The chemical symbol for Hydrogen is H.
How are atomic nuclei determined?
Properties of atomic nuclei (atomic mass, nuclear cross-sections) are determined by the number of protons and number of neutrons (neutron number). It must be noted, especially nuclear cross-sections may vary by many orders from nuclide with the neutron number N to nuclide with the neutron number N+1. For example, actinides with odd neutron number are usually fissile (fissionable with slow neutrons) while actinides with even neutron number are usually not fissile (but are fissionable with fast neutrons). Heavy nuclei with an even number of protons and an even number of neutrons are (due to Pauli exclusion principle) very stable thanks to the occurrence of ‘paired spin’. On the other hand, nuclei with an odd number of protons and neutrons are mostly unstable.
What are the two forces that make up the nucleus?
Atomic nuclei consist of protons and neutrons, which attract each other through the nuclear force, while protons repel each other via the electric force due to their positive charge. These two forces compete, leading to various stability of nuclei. There are only certain combinations of neutrons and protons, which forms stable nuclei.
What is the charge of an atom?
Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. In a neutral atom there are as many electrons as protons moving about nucleus. It is the electrons that are responsible for the chemical bavavior of atoms, and which identify the various chemical elements.
What is the first ionization energy of aluminum?
First Ionization Energy of Aluminium is 5.9858 eV.
How many protons does aluminum have?
Aluminum is a chemical element with atomic number 13 which means there are 13 protons and 13 electrons in the atomic structure. The chemical symbol for Aluminum is Al.
What is the atomic mass of 12 C?
For 12 C the atomic mass is exactly 12u , since the atomic mass unit is defined from it. For other isotopes, the isotopic mass usually differs and is usually within 0.1 u of the mass number. For example, 63Cu (29 protons and 34 neutrons) has a mass number of 63 and an isotopic mass in its nuclear ground state is 62.91367 u.
What is the unit of mass?
The unit of measure for mass is the atomic mass unit (amu). One atomic mass unit is equal to 1.66 x 10 -24 grams. One unified atomic mass unit is approximately the mass of one nucleon (either a single proton or neutron) and is numerically equivalent to 1 g/mol.
Which atom is the least electronegative?
The most electronegative atom, fluorine, is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7.
Which is heavier, a proton or a neutron?
The neutron is slightly heavier than the proton. This increases the mass of nuclei with more neutrons than protons relative to the atomic mass unit scale based on 12 C with equal numbers of protons and neutrons.
What is the symbol for electronegativity?
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. For this purposes, a dimensionless quantity the Pauling scale, symbol χ, is the most commonly used.
