How do you calculate average atomic mass?
Part 1 Part 1 of 2: Calculating Average Atomic Mass Download Article
- Understand isotopes and atomic masses. Most elements can naturally occur in multiple forms, or isotopes.
- Look up the mass of each isotope. You'll need two pieces of information for each isotope, which you can look up in a reference book or an online source ...
- Write down the abundance of each isotope. ...
- Turn your abundance percentages into decimals. ...
What is the formula for average atomic mass?
Atomic mass = Number of protons + number of neutrons + number of electrons. In other words, when proton, electrons, and neutrons of one atom are added together then this is named as average mass of the atom. As compared to neutrons and the protons, electrons are so limited in count that it has only a small affect on the calculation.
What is the atomic number and mass number of bromine?
Mass numbers of typical isotopes of Bromine are 79; 81. Atomic mass of Bromine is 79.904 u. The atomic mass is the mass of an atom. The atomic mass or relative isotopic mass refers to the mass of a single particle, and therefore is tied to a certain specific isotope of an element.
How to find the molar mass of bromine?
Molar mass of BrF3 = 136.8992096 g/mol. Convert grams Bromine Trifluoride to moles or moles Bromine Trifluoride to grams. Molecular weight calculation: 79.904 + 18.9984032*3.
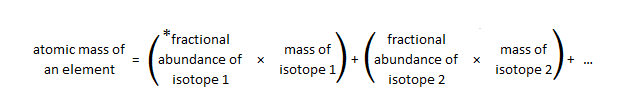
What are the atomic mass of bromine isotopes?
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and an atomic mass of 79.904 amu.
How do you calculate the average atomic mass of bromine isotopes?
Calculate The Average Atomic Mass Of Bromine AtomAnswer: The atomic mass of an element is the mass of one atom of that element. ... ⇒ 40.64u. Hence, average atomic mass of the bromine atom = 39.26 + 40.64 u = 79.9u.Answer. The average atomic mass of the bromine atom = 79.9u.
What is the average atomic number of bromine?
Bromine is a chemical element with symbol Br and atomic number 35. Classified as a halogen, Bromine is a liquid at room temperature.
What is the average atomic mass of an isotope?
0:127:19How To Calculate The Average Atomic Mass - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe're given the percent abundance of each isotope. And also the atomic mass of those two isotopes.MoreWe're given the percent abundance of each isotope. And also the atomic mass of those two isotopes. So using that data how can we calculate the average atomic mass of chlorine. Now it turns out that
How do you calculate the atomic mass of an isotope?
Use the atomic masses of each of the isotopes along with their percent abundances to calculate the average atomic mass. Change each percent abundance into decimal form by dividing by 100. Multiply this value by the atomic mass of that isotope. Add together for each isotope to get the average atomic mass.
How do you find the atomic mass of two isotopes?
To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
How many atoms are in an isotope?
An isotope is one of two or more species of atoms of a chemical element with the same atomic number and position in the periodic table and nearly identical chemical behavior but with different atomic masses and physical properties. Every chemical element has one or more isotopes.
How do you find the average mass of an isotope in Class 9?
Hint: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
What is the meaning of average atomic mass?
The average atomic mass (sometimes called atomic weight) of an element is the weighted average mass of the atoms in a naturally occurring sample of the element. Average masses are generally expressed in unified atomic mass units (u), where 1 u is equal to exactly one-twelfth the mass of a neutral atom of carbon-12.
Why is the atomic mass of an element an average atomic mass?
Explanation: The mass written on the periodic table is an average atomic mass taken from all known isotopes of an element. This average is a weighted average, meaning the isotope's relative abundance changes its impact on the final average. The reason this is done is because there is no set mass for an element.
How do you find the mass of isotope abundance?
How do you calculate the percentage of abundance based on mass? Calculate the average atomic mass using the atomic masses of each isotope and their percent abundances. Divide each percent abundance by 100 to convert it to decimal form. Multiply this value by the isotope's atomic mass.
What is the difference between atomic mass and average atomic mass?
The key difference between atomic mass and average atomic mass is that the atomic mass is the mass of an atom, whereas the average atomic mass is the mass of an atom of a particular chemical element calculated by considering isotopes of that element.
What is the average atomic mass of bromine 79 and bromine 81?
Bromine has two naturally occurring isotopes (Br-79 and Br-81) and an atomic mass of 79.904 amu. The mass of Br-81 is 80.9163 amu, and its natural abundance is 49.31%.
What is the average mass of bromine from the following data BR 79 50.69 %) and BR-81 49.31 %)?
One isotope of bromine has an atomic mass of 78.92amu and a relative abundance of 50.69%. The other major isotope of bromine has an atomic mass of 80.92amu and a relative abundance of 49.31%.
How do you find the average mass of an isotope in Class 9?
Hint: To calculate the average atomic mass, multiply the fraction by the mass number for each isotope, then add them together.
How do you calculate isotopic abundance?
To calculate the percent abundance of each isotope in a sample of an element, chemists usually divide the number of atoms of a particular isotope by the total number of atoms of all isotopes of that element and then multiply the result by 100.