
What is bioavailability and why does it matter?
Low bioavailability is commonly caused by, among other things, age, sex, physical activity levels, stress levels, genetic phenotypes. Why is bioavailability important? Bioavailability is important from a research perspective, because it provides key data related to how compounds engage with the body through their delivery methods.
What does the term 'bioavailability' mean?
What does the term bioavailability mean? Bioavailability has been broadly defined as the “absorption and utilisation of a nutrient”. 1 This means that it is all very well taking a drug or a nutrient, but what really matters is how well we can absorb the substance and then how well can our body make good use of it.
What is IV drug use, what drugs?
Intravenous drug use involves injecting a substance into a vein using a syringe. This method of administration produces rapid and heightened effects because it bypasses the process of first pass metabolism that all orally administered drugs undergo, in which the drug must first be absorbed in the intestines, carried to the liver and subjected ...
What is total bioavailability?
What is Total Bioavailability? Total Bioavailability™ means the degree to which an active ingredient becomes available for its desired purpose after entering the body. It has two components: Functional bioavailability, , the amount that actually exerts its health-enhancing effects.

What does 70% bioavailability mean?
When a medicine is given orally, only part of the administered dose appears in the plasma. (Example: if 100 mg of a medicine are administered orally and 70 mg of this medicine are absorbed unchanged, the bioavailability is 0.7 or seventy percent).
How is bioavailability of a drug measured?
For drugs excreted primarily unchanged in urine, bioavailability can be estimated by measuring the total amount of drug excreted after a single dose. Ideally, urine is collected over a period of 7 to 10 elimination half-lives for complete urinary recovery of the absorbed drug.
Why is drug bioavailability important?
Bioavailability is a key indicator of drug absorption. It represents the administered dose fraction which achieves success in reaching the systemic circulation when administered orally or through any other extravascular dosing route.
What is bioavailability of an oral drug?
Drug oral bioavailability is the fractional extent of the drug dosage that finally reaches the therapeutic site of action and is quantitatively symbolized as %F (1). In many cases, most of the orally administered drug is metabolized and eliminated before reaching systemic blood circulation (1).
Which drug has highest bioavailability?
Drugs given by intravenous route have 100% bioavailability.
What are the 2 types of bioavailability?
Types of bioavailability are as follow:Absolute Bioavailability: When the drug is administered through the intravenous route, the bioavailability of the drug achieved will be 100 percent. ... Relative Bioavailability: It is the bioavailability of the drug when obtained and it is compared with a reference standard.More items...
What factors affect drug bioavailability?
Drug bioavailability after oral administration is affected by anumber of different factors, including physicochemical properties of the drug, physiological aspects, the type of dosage form, food intake, biorhythms, and intra- and interindividual variability of the human population.
What is bioavailability with example?
(BY-oh-uh-VAY-luh-bul) The ability of a drug or other substance to be absorbed and used by the body. Orally bioavailable means that a drug or other substance that is taken by mouth can be absorbed and used by the body.
What increases bioavailability?
The bioavailability can be improved with the use of different novel delivery systems like liposomes, marinosomes, niosomes and lipid based systems which can enhance the rate of release as well as the capacity to cross the lipid rich biomembranes[4].
What does high bioavailability mean?
A higher bioavailability generally means more nutrients are being absorbed than through conventional methods. A number of different influences can affect this, from timing, age, sex and lifestyle to the format used to take the supplement and when it was taken in the day i.e. before a meal.
Can bioavailability be more than 100 %?
Bioavailability more than 1 (more than 100%) is a common phenomena, not only in i.p. and Sc but also in oral administration. F greater than 1 is relative to i.v. administration and assuming that in any of the routes compared, Clearance does not change.
What does low bioavailability mean?
What does poor bioavailability mean? Poor, or low bioavailability is often a feature of compounds that have poor water-solubility and/or are slowly absorbed. This means that they aren't efficiently absorbed in the gastrointestinal (GI) tract before wending their way out of the other end with all the waste products.
How is bioavailability expressed?
Bioavailability is expressed as the percentage of the total drug dose administered that reaches the circulation. For a drug taken orally, the 'first-pass effect' of hepatic metabolism reduces bioavailability.
Which is the method of choice in bioavailability determination?
The in-vivo bioavailability of a drug product is demonstrated by the rate and extent of drug absorption, as determined by comparison of measured parameters, e.g., concentration of the active drug ingredient in the blood, cumulative urinary excretion rates, or pharmacological effects.
Can bioavailability be more than 100 %?
Bioavailability more than 1 (more than 100%) is a common phenomena, not only in i.p. and Sc but also in oral administration. F greater than 1 is relative to i.v. administration and assuming that in any of the routes compared, Clearance does not change.
How do you measure bioequivalence?
Bioequivalence is determined based on the relative bioavailability of the innovator medicine versus the generic medicine. It is measured by comparing the ratio of the pharmacokinetic variables for the innovator versus the generic medicine where equality is 1.
What is bioavailability in medicine?
Bioavailability refers to the extent a substance or drug becomes completely available to its intended biological destination(s). More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; the site of action or the bodily fluid domain from which the drug’s intended targets ...
What is the definition of bioavailability?
Bioavailability refers to the extent a substance or drug becomes completely available to its intended biological destination(s). More accurately, bioavailability is a measure of the rate and fraction of the initial dose of a drug that successfully reaches either; the site of action or the bodily flu …
Why is bioavailability based on peak plasma concentration misleading?
Bioavailability determinations based on the peak plasma concentration can be misleading because drug elimination begins as soon as the drug enters the bloodstream. Peak time (when maximum plasma drug concentration occurs) is the most widely used general index of absorption rate; the slower the absorption, the later the peak time.
Why is bioavailability low?
Low bioavailability is most common with oral dosage forms of poorly water-soluble, slowly absorbed drugs. Insufficient time for absorption in the gastrointestinal (GI) tract is a common cause of low bioavailability.
What is the most reliable measure of a drug's bioavailability?
The most reliable measure of a drug’s bioavailability is AUC. AUC is directly proportional to the total amount of unchanged drug that reaches systemic circulation. Drug products may be considered bioequivalent in extent and rate of absorption if their plasma concentration curves are essentially superimposable.
What is bioavailability in medicine?
Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Differences in bioavailability among formulations ...
How to determine bioavailability of a drug?
For drugs excreted primarily unchanged in urine, bioavailability can be estimated by measuring the total amount of drug excreted after a single dose. Ideally, urine is collected over a period of 7 to 10 elimination half-lives for complete urinary recovery of the absorbed drug. After multiple dosing, bioavailability may be estimated by measuring unchanged drug recovered from urine over a 24-hour period under steady-state conditions .
What chemical reaction reduces absorption?
They include formation of a complex (eg, between tetracycline and polyvalent metal ions), hydrolysis by gastric acid or digestive enzymes (eg, penicillin and chloramphenicol palmitate hydrolysis), conjugation in the intestinal wall (eg, sulfoconjugation of isoproterenol ), adsorption to other drugs (eg, digoxin to cholestyramine), and metabolism by luminal microflora.
What is therapeutic equivalence?
Therapeutic equivalence indicates that drug products, when given to the same patient in the same dosage regimen, have the same therapeutic and adverse effects. Bioequivalent products are expected to be therapeutically equivalent. Therapeutic nonequivalence (eg, more adverse effects, less efficacy) is usually discovered during long-term treatment ...
What chemical reaction reduces absorption?
Chemical reactions that reduce absorption can decrease bioavailability. They include formation of a complex (eg, between tetracycline and polyvalent metal ions), hydrolysis by gastric acid or digestive enzymes (eg, penicillin and chloramphenicol palmitate hydrolysis), conjugation in the intestinal wall (eg, sulfoconjugation of isoproterenol ), ...
Why is bioavailability based on peak plasma concentration misleading?
Bioavailability determinations based on the peak plasma concentration can be misleading because drug elimination begins as soon as the drug enters the bloodstream. Peak time (when maximum plasma drug concentration occurs) is the most widely used general index of absorption rate; the slower the absorption, the later the peak time.
Why is bioavailability low?
Low bioavailability is most common with oral dosage forms of poorly water-soluble, slowly absorbed drugs. Insufficient time for absorption in the gastrointestinal (GI) tract is a common cause of low bioavailability.
What is the most reliable measure of a drug's bioavailability?
The most reliable measure of a drug’s bioavailability is AUC. AUC is directly proportional to the total amount of unchanged drug that reaches systemic circulation. Drug products may be considered bioequivalent in extent and rate of absorption if their plasma concentration curves are essentially superimposable.
What is bioavailability in medicine?
Bioavailability refers to the extent and rate at which the active moiety (drug or metabolite) enters systemic circulation, thereby accessing the site of action. Bioavailability of a drug is largely determined by the properties of the dosage form, which depend partly on its design and manufacture. Differences in bioavailability among formulations ...
What is therapeutic equivalence?
Therapeutic equivalence indicates that drug products, when given to the same patient in the same dosage regimen, have the same therapeutic and adverse effects. Bioequivalent products are expected to be therapeutically equivalent. Therapeutic nonequivalence (eg, more adverse effects, less efficacy) is usually discovered during long-term treatment ...
How to determine bioavailability of a drug?
For drugs excreted primarily unchanged in urine, bioavailability can be estimated by measuring the total amount of drug excreted after a single dose. Ideally, urine is collected over a period of 7 to 10 elimination half-lives for complete urinary recovery of the absorbed drug. After multiple dosing, bioavailability may be estimated by measuring unchanged drug recovered from urine over a 24-hour period under steady-state conditions .
What is the role of CYP 450 enzymes in the body?
CYP 450 enzymes play an important role in hepatic drug metabolism. Prodrugs – and what differentiates a prodrug from a drug. A prodrug is an inactive drug that needs to be converted to its active form by enzymes in the body, such as hepatic CYP 450 enzymes.
What is the first pass effect?
This is known as the first-pass effect. Drug must first-pass through the liver before it finally reaches the systemic bloodstream. There is a wide range of important enzymes in the liver – known as CYP 450 enzymes – that interact with and metabolize drugs.
What is bioavailability in pharmacy?
Bioavailability is one of the many concepts pharmacy technicians are expected to know. It is one of the topics stated on the PTCB syllabus for the 2020 exam. Students must, therefore, have a solid understanding of this fundamental concept.
Why are drugs administered via intravenous route almost or near 100 percent bioavailability?
Drugs administered via the intravenous route have an almost or near 100 percent bioavailability. That is because there are almost no barriers to entry. Compare that to the process of what orally administered drugs must go through. Drugs administered orally are under assault from:
What percentage of Amoxicillin is bioavailable?
Amoxicillin has a bioavailability of 95 percent – meaning 95 percent of the drug reaches the systemic circulation. 5 percent of the active ingredient does not make it. The anti-depressant medicine, Prozac (fluoxetine), has a lower bioavailability, of approximately 70 percent – meaning 30 percent was metabolized/removed by ...
What are the factors that influence how a drug reacts with the body?
Factors that influence how a drug reacts with the body include: Physical properties of the drug – such as solubility and hydrophobicity (its propensity to repel against water; think of oil and water, for example – oil has a high degree of “hydrophobicity”).
What is the concept of bioavailability?
The concept of bioavailability; the fraction of drug that reaches the systemic circulation after undergoing metabolism.
What is bioequivalence in drug testing?
Bioequivalence is concluded if the average bioavailability of the test formulation is within ±20% of that of the reference formulation with a certain assurance. This decision rule is based on the additive model and not on relative or percent change. Thus, it is not employed commonly for most drug products.
How is bioequivalence assessed?
A typical process for bioequivalence assessment is to conduct a bioequivalence study with male healthy volunteers under the assumption that bioequivalence (relative bioavailability) of the drug product under investigation is predictive of clinical outcomes (i.e. , safety and efficacy) of the drug product in clinical trials. A bioequivalence study is often conducted utilizing a crossover design that allows comparison within individual subjects, i.e., each subject is at his/her own control. Based on pharmacokinetic (PK) data collected, bioequivalence can then be assessed using valid statistical methods according to some pre-specified regulatory criteria for bioequivalence. As indicated by the FDA, an approved generic drug product can be used as a substitute for the brand-name drug.
What is the bioequivalence limit for a multiplicative model?
For the multiplicative model, we consider the (0.8, 1.25) as the bioequivalence limit for δ= μT/μRand μTand μRare the median bioavailabilities of the test and reference formulations, respectively. Similarly, the sample size nrequired to achieve a 1 – βpower at the αnominal level for each corresponding design kafter the logarithmic transformation is determined by the following equations [10-11]:
What is the average bioequivalence?
Bioequivalence is concluded if the average bioavailability of the test formulation is within (80%, 125%) that of the reference formulation , with a certain assurance. From a multiplicative model for pharmacokinetic responses, the FDA 2003 guidance suggests that the logarithmic transformation on AUC(0-∞) or AUC(0-tlast) and Cmaxbe considered [6]. As a result, this criterion is not symmetric about 1 on the original scale where the maximum probability of concluding average bioequivalence occurs. However, on the logarithmic scale, the criterion has a range of – 0.2231 to 0.2231, which the symmetric about 0 where the probability of concluding average bioequivalence is at maximum. Current FDA regulation adopts the 80/125 rule after log-transformation. That is, two drug products are said to be (average) bioequivalence (ABE) if the 90%confidence interval of the ratio of geometric meansof the primary pharmacokinetic (PK) responses (after log-transformation) is within the bioequivalence limits of 80%and 125%.
What is bioavailability in medicine?
Bioavailability is referred to as the extent and rate to which the active drug ingredient or active moiety from the drug product is absorbed and becomes available at the site of drug action. The relative bioavailability in terms of the rate and extent of drug absorption is considered predictive of clinical outcomes. In 1984, the United States Food and Drug Administration (FDA) was authorized to approve generic drug products under the Drug Price Competition and Patent Term Restoration Actbased on evidence of average bioequivalence in drug absorption through the conduct of bioavailability and bioequivalence studies. This article provides an overview (from an American point of view) of definition of bioavailability and bioequivalence, Fundamental Bioequivalence Assumption, regulatory requirements, and process for bioequivalence assessment of generic drug products. Basic considerations including criteria, study design, power analysis for sample size determination, and the conduct of bioequivalence trial, and statistical methods are provided. Practical issues such as one size-fits-all criterion, drug interchangeability and scaled average criteria for assessment of highly variable drug products are also discussed.
What is an ANDA for a drug?
When an innovative (or brand-name) drug product is going off patent, pharmaceutical or generic companies may file an abbreviated new drug application (ANDA) for generic approval. Generic drug products are defined as drug products that are identicalto an innovative (brand-name) drug which is the subject of an approved NDA with regard to active ingredient(s), route of administration, dosage form, strength, and conditions of use. Since ANDA submissions for generic applications do not require lengthy clinical evaluation of the generic drugs under investigation (see Table 1), the price of generics are usually much lower than that of the originals. On average, it is about 20% of the price of the brand-name originals. In 1984, the United States Food and Drug Administration (FDA) was authorized to approve generic drug products under the Drug Price Competition and Patent Term Restoration Act. The purpose is to make less expensive, safe, and equally efficacious generics available to general public after the expiration of patent protection of expensive brand-name drugs. For approval of generic drug products, the FDA requires that evidence of average bioequivalence in drug absorption be provided through the conduct of bioavailability and bioequivalence studies. Bioequivalence assessment is considered as a surrogate for clinical evaluation of the therapeutic equivalence of drug products.
What is Williams design?
Williams’ design is a variance stabilizing design. More information regarding the construction and good design characteristics of Williams’ designs can be found in [1].
Bioavailability Definition
- The bioavailability of drugs describes the rate and extent to which drugs are absorbed from their sites of administration and become available at the sites of action. Drugs that are administered orally have low bioavailability due to incomplete absorption and first-pass metabolism in the liver, resulting in the oral bioavailability of drugs being a...
The Basics of Bioavailability
- Before learning what bioavailability of drugs is, you should learn a little bit about absorption, distribution, metabolism and excretion (ADME). The ADME process serves as a filter for all foreign compounds in your body and determines how much remains to be metabolized by your liver. Not all drugs are absorbed equally; for example, some drugs are more soluble in water than others. …
Bioavailability Example
- The bioavailability of a drug refers to how much of it actually gets into your bloodstream and is available to be used by your body. The amount that gets into your bloodstream depends on how well it absorbs into your digestive system and gets absorbed from there into other parts of your body. This process can be affected by what you’ve recently eaten, how much water you’ve had w…
Importance of Bioavailability
- If you’re trying to be treated with medications and other drugs, then it’s important to know what bioavailability means. The bioavailability of a drug refers to how much of a drug is absorbed by your body when ingested. In many cases, bioavailability varies from patient to patient and can even vary from dose to dose for one individual. It also depends on multiple factors, including wh…
Low Bioavailability
- Some medications have a low bioavailability, which means that they are poorly absorbed in your digestive tract. If a drug has a low bioavailability, you may need to take multiple doses throughout the day or change your dosing schedule. You might also want to consider switching medications if one has such low bioavailability that it’s not helping or causing negative side effects. Drugs lik…
How to Improve Drug Bioavailability
- There are many methods for improving drug bioavailability. Some of these methods include changing a drug’s formulation, altering an animal’s diet, and even administering drugs in combination with other compounds. For example, researchers have found that feeding animals atrazine—a commonly used herbicide that can inhibit thyroid hormone production—can significa…
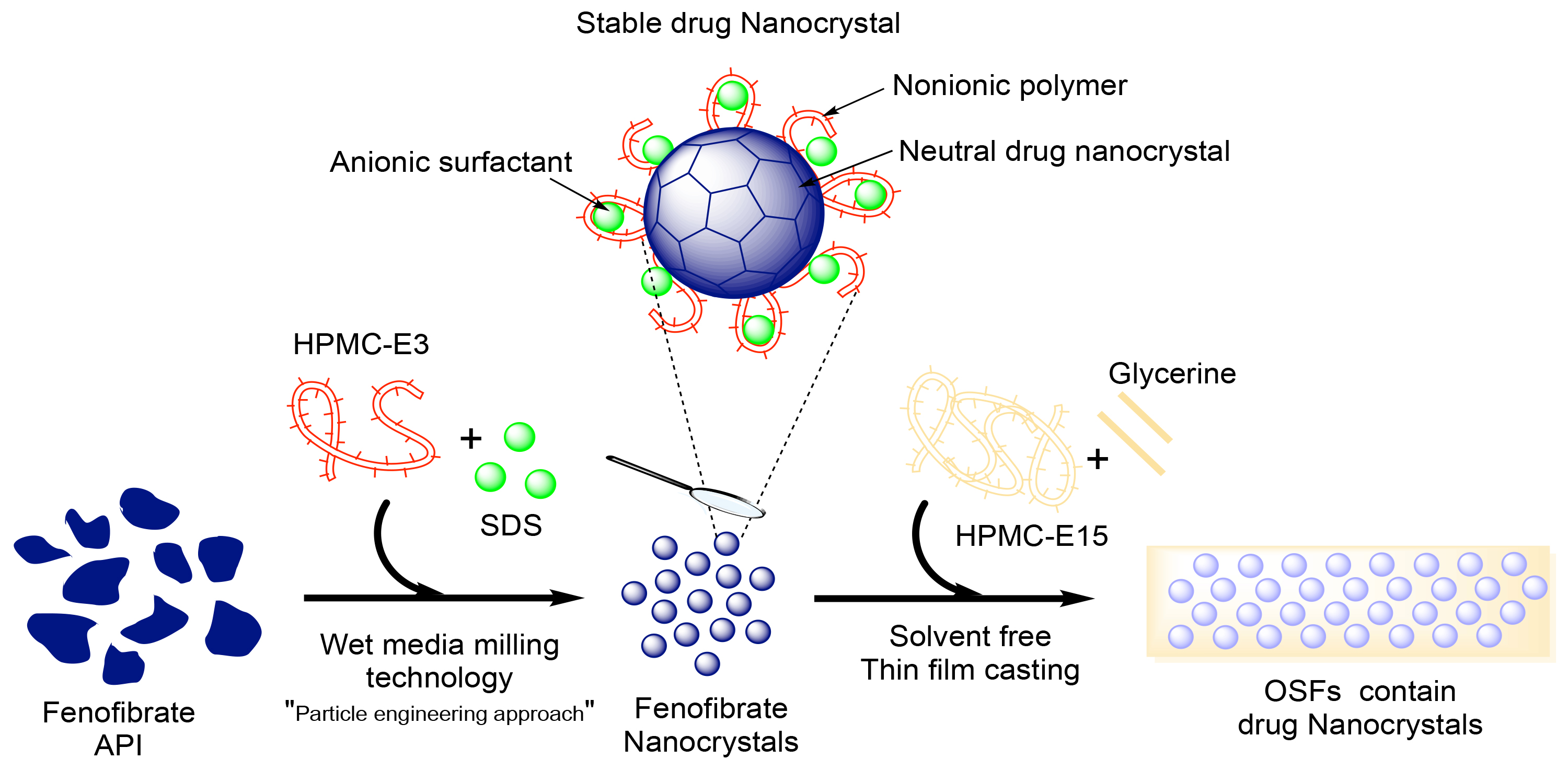
Improving The Bioavailability with Nanotechnology
- New research shows that improved bioavailability could be a matter of packaging. At the University at Buffalo, in New York, and Peking University, in Beijing, scientists have developed a gel-based delivery system that makes drugs more easily absorbed by body tissue. These nanogels mimic cell membranes, so once they’re absorbed by your gut or blood vessels—they send drugs …
Future Considerations
- It’s important to consider what will happen to a drug once it makes its way into your body. How much of it will be absorbed? Where will it go and how long will it stay there? With so many different factors coming into play, bioavailability is incredibly important. The bioavailability of a drug refers to how much of an active ingredient reaches systemic circulation and is therefore av…