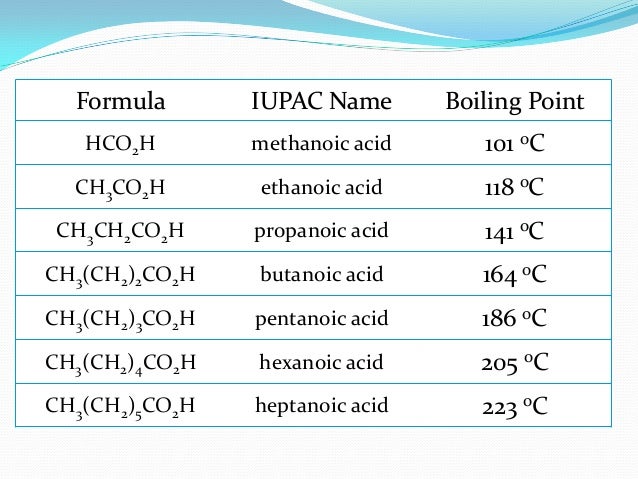
Boiling point of some common organic compounds
| Compound | Boiling Point ( o C) | Compound | Boiling Point ( o C) |
| Benzyl Alcohol | 205 | Ethyl Benzoate | 213 |
| Glycerol | 290 | Methyl Salicylate | 223 |
| Ethylene Glycol | 197 | Nitrobenzene | 211 |
| Phenol | 182 | Aniline | 184 |
| Compound | Boiling Point (oC) |
|---|---|
| Benzyl Alcohol | 205 |
| Glycerol | 290 |
| Ethylene Glycol | 197 |
| Phenol | 182 |
How do you determine boiling points of compounds?
You can determine which molecule has the higher boiling point by knowing which bonds require more energy in order for the gas phase to be achieved. Rated in order from strongest to weakest these forces are: Ionic > Hydrogen bond > Dipole > van der Waals forces. Functional groups are also indicators.
Do organic compounds have a high or low melting point?
Organic compounds have relatively high melting and boiling point when compared to inorganic compounds that generally have a low meting and boiling points. Organic compounds are biological and more complex in nature when compared to inorganic compounds that are simple and mineral in nature.
How does polarity affect boiling point of organic compounds?
Principle: The greater the forces of attraction the higher the boiling point or the greater the polarity the higher the boiling point. The other molecules are slightly polar and show the increase in boiling point with molecular weight which is normal. Instead, water boils at +100 C, which is very abnormal.
Why do organic compounds have a melting point range?
The melting point determination of organic compounds helps people understand the physical and chemical properties of the substance. Many different factors affect the melting point of any substance, such as the force of attraction, impurities present in the substance, and the molecules' size and structure.
Does organic compounds have high boiling point?
Organic compounds can be gases, liquids, or solids at room temperature. Ionic compounds are all solids at room temperature with very high melting points. Organic compounds have relatively low melting points and boiling points.
Is organic compound low boiling point?
Boiling points Hydrocarbons are non-polar, so there is only weak dispersion forces between molecules. This means that the boiling points are relatively low. Longer chains of hydrocarbons have a greater boiling points. This is due to more dispersion force between the molecules.
How do you know which organic compound has a higher boiling point?
As a rule, larger molecules have higher boiling (and melting) points. Consider the boiling points of increasingly larger hydrocarbons. More carbons and hydrogens means a greater surface area possible for van der Waals interaction, and thus higher boiling points.
What is melting or boiling point range of organic compound?
The compounds in solid form have to be overcome by heating at a temperature for them to lose their intermolecular forces that hold solid together. Crystalline organic compounds (Pure and Nonionic) have a very sharp and characteristic melting point of 0.5 to 1.0⁰C range.
Which has higher boiling point organic or inorganic?
Inorganic compounds are mostly made of strong ionic bonds, which give them a very high melting and boiling point. On the other hand, organic compounds are made of comparatively weak covalent bonds, which is the cause of their low melting and boiling point.
How do you determine boiling point of compounds?
Intermolecular forces (IMFs) can be used to predict relative boiling points. The stronger the IMFs, the lower the vapor pressure of the substance and the higher the boiling point. Therefore, we can compare the relative strengths of the IMFs of the compounds to predict their relative boiling points.
How is boiling point determined in organic chemistry?
The boiling point of organic compounds depends on their molecular weight. As the molecular weight increases, so does the boiling point. For two compounds of the same molecular weight, various factors determine the boiling point of an organic compound.
Which organic compound has the lowest boiling point?
Ethene has lowest boiling point. The boiling points of ethane, ethene and ethyne are 184.5 K, 171 K and 198 K respectively.
Which compound has higher boiling point?
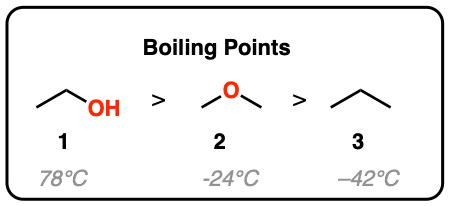
Ethanol has the highest boiling point (C2H5OH) because of higher description or vander waal forces and dipole-dipole interactions.
What property of organic compounds decreases boiling point?
Branching decreases the boiling point So the increase of surface area increases the ability of individual molecules to attract each other. Branching in molecules decreases the surface area thereby decreasing the attractive force between individual molecules. As a result, the boiling point decreases.
What is melting point in organic chemistry?
Melting point (mp): The temperature (or more commonly temperature range) at which a substance undergoes a solid to liquid phase change (i.e., it melts) without an increase in temperature. Alternately, the temperature at which a substance exists in equilibrium between its solid and liquid phases.
Why do organic compounds have low melting points?
Organic compounds have a low melting point because of weaker bond strength of bonds present in between the atoms of the compound.
Why organic compounds have low boiling point?
Explanation: Organic substances have a relatively low polarity and generally have weaker intermolecular forces to hold them together tightly.
What types of compounds have the lowest melting boiling point?
Covalent compounds have generally low melting and boiling points. (i) are bad conductors of electricity. (ii) have low melting and boiling points.
Do inorganic compounds have high melting points?
Inorganic compounds are often ionic, and so have very high melting points.
What are properties of organic compound?
The general characteristics of Organic Compounds are:Organic compounds include complex structures and high molecular weights.These are soluble in organic solvents and mostly insoluble in water.Mostly depend on only three elements: Carbon, Hydrogen and nitrogen.These compounds are combustible in nature.More items...
1. What is the boiling point of Benzene, water and Benzaldehyde?
Boiling points of Benzene, water and Benzaldehyde are 780 C, 1000 C and 1780 C respectively.
2. What do you understand about the boiling point of the compound?
The boiling point of an organic molecule is the temperature at which an attractive force (such as Vanderwaal attractive force) or an intermolecular...
3. What is the main factor that determines the boiling point of an organic compound?
The most important factor is the shape of the molecule first, whether or not the molecule is branched. If the molecule is more branched, it means a...
4. What are a few precautions that need to be taken during the experiment?
Make sure that the capillary tube is closed properly and that the seal point of the capillary tube should be well immersed in the liquid.
5. How does the polarity of Organic compounds affect the boiling point?
The polarity of a molecule determines the attractive force between molecules in a liquid state. In polar compounds, it is seen that the positive si...
6. Explain why carboxylic acids have a higher boiling point than hydrocarbons.
Carboxylic acids have a higher boiling point as they have the capacity to form hydrogen bonds. These bonds provide these molecules with extra stabi...
7. What would be the boiling point of water at higher altitudes?
At higher altitudes, the boiling point would be less than 1000 C.
What is boiling point in chemistry?
The Meaning of Boiling Point. There are some topics in Chemistry that are very important from the point of view of the final examinations. The topic of determination of the boiling point of an organic compound is one such topic. The determination of the boiling point of organic compounds is a topic that can also come up during the practice exam ...
What is the boiling point of a substance?
In the simplest terms, the boiling point of a substance can be defined as a particular temperature at which that substance would achieve a vapour pressure that is equal to the pressure around the liquid. This is also the temperature at which the liquid will change into a vapour. To a large extent, the boiling point of a liquid will be dependent on ...
How to find boiling point of benzene?
The Procedure for Finding Out the Boiling Point of Benzene 1 The student should begin by taking the capillary tube and closing its end by holding the end in the flame. Rotating the tube for 2-3 minutes should do the trick 2 A few millilitres of benzene should be transferred to the fusion tube 3 Proceed to dip the capillary tube in the liquid in the fusion tube. Do not forget to keep the sealed end of the tube up 4 The tube should be inserted in one of the holes of the aluminium block. After that, a thermometer should also be inserted in the same block 5 After everything is placed inside the hole, one should make sure that the liquid is completely visible in the fusion tube 6 Put the aluminium block on the tripod 7 Use the kerosene burner to slowly heat the aluminium block 8 Make a note of the temperature as soon as regular streams of bubbles appear in the liquid present in the fusion tube
What happens to the boiling point of a liquid when it is in a partial vacuum?
This means that if a liquid is in a partial vacuum, then it would have a lower boiling point in comparison to the boiling point ...
Why do carboxylic acids have a higher boiling point than hydrocarbons?
Explain why carboxylic acids have a higher boiling point than hydrocarbons. Carboxylic acids have a higher boiling point as they have the capacity to form hydrogen bonds.
What is organic chemistry?
Students must be aware of the fact that organic chemistry is a branch of chemistry that mainly deals with the subject of composition and synthesis of organic chemical compounds. For students who are not familiar with the term, organic compounds are compounds that contain a carbon atom in their composition.
Which element has the lowest boiling point?
Did you know that the element with the lowest boiling point in the world is helium? Further, the boiling points of tungsten and rhenium are said to exceed 5000 K, but nobody has been able to find out the exact boiling point as it is difficult to measure extreme temperatures.
What does the boiling point of a compound mean?
The normal boiling point of a compound is an indicator of the volatility of that compound. The higher the boiling point, the less volatile is the compound.
What happens when the boiling point of a compound is higher?
If the boiling point of the compound is higher, it then exists as a liquid or a solid.
Why is the boiling point of butanol higher than that of butanol?
But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond.
How does branching affect the boiling point?
Branching decreases the boiling point. As the length of carbon chain increases, the surface area of the compound will also increase. Van der Waals dispersion force is proportional to the surface area. So the increase of surface area increases the ability of individual molecules to attract each other. Branching in molecules decreases the surface ...
What forces affect the boiling point of a compound?
The relative strength of intermolecular forces such as ionic, hydrogen bonding, dipole-dipole interaction and Vander Waals dispersion force affects the boiling point of a compound. The influence of these forces depends on the functional group present. We can explain the effect of these forces on ...
What are the derivatives of butane?
Consider butane and its three derivatives such as diethyl ether, n- butanol and sodium n- butoxide.
What is the boiling point of diethyl ether?
In the case of diethyl ether, the molecules are held together by dipole-dipole interaction which arises due to the polarized C-O bond. Its boiling point is 35 o C. Compare its boiling point with that of n-butanol. The boiling point of n-butanol is 117 o C.
What does the boiling point of a compound mean?
The normal boiling point of a compound is an indicator of the volatility of that compound. The higher the boiling point, the less volatile is the compound.
What happens when the boiling point of a compound is higher?
If the boiling point of the compound is higher, it then exists as a liquid or a solid.
What determines the force of attraction between the molecules in the liquid state?
Polarity of the molecule determines the force of attraction between the molecules in the liquid state. In polar compounds, the positive end of one molecule is attracted by the negative end of another molecule. That means polar molecules are attracted by opposite charge effect.
What forces affect the boiling point of a compound?
The relative strength of intermolecular forces such as ionic, hydrogen bonding, dipole-dipole interaction and Vander Waals dispersion force affects the boiling point of a compound. The influence of these forces depends on the functional group present.
Why is the boiling point of butanol higher than that of butanol?
But the boiling point of sodium butoxide is higher than that of butanol because the attractive force in sodium butoxide is very strong ionic bond.
How does branching affect the boiling point?
Branching decreases the boiling point. As the length of carbon chain increases, the surface area of the compound will also increase. Van der Waals dispersion force is proportional to the surface area. So the increase of surface area increases the ability of individual molecules to attract each other. Branching in molecules decreases the surface ...
What is the kinetic energy of a molecule?
Kinetic energy depends on the temperature, mass and velocity of a molecule. When the temperature increases, the average kinetic energy of particles also increases. When the temperature reaches the boiling point, the average kinetic energy becomes sufficient to overcome the force of attraction between the liquid particles.
Which functional group elevates boiling points?
Basically, Polar functional groups that are more exposed will elevate boiling points to a greater extent. (This applies for aldehydes, ketones and alcohols.)
Which alkane has more surface?
A longer alkane has more surface and thus can stick more effectively to other alkane molecules.
What is the boiling point of a compound?
The definition of boiling point states that it is the temperature when the vapor pressure of the compound equals to the atmospheric pressure. In other words, we can say it is the temperature at which there is so much of the compound evaporated that it creates a pressure equal to the external pressure. In a simpler perspective let’s say that it is ...
What factors influence the boiling point of a molecule?
The larger this surface, the stronger the intermolecular interactions, and thus, the higher the boiling point. This can be seen by comparing the boiling points of pentane, 2-methylbutane, and 2,2-dimethylpropane:
Why does melting point depend on the shape of the molecules?
Aside from the intermolecular interactions , however, the melting point depends also on how the molecules are packed or arranged in the solid form. The more symmetrical they are, the better they pack and form a perfect crystal lattice which results in a higher melting point. So, as the molecules fit tighter, more energy is required to break the lattice and melt them apart.
Why does the boiling point increase when going from the tertiary to the primary amine?
Notice that the boiling point increases as we are going from the tertiary to the primary amine as a result of increasing the number of hydrogen bonding per nitrogen. Propylamine has two hydrogens connected to the nitrogen and each can make a hydrogen bond with a neighboring nitrogen atom from another molecule. This makes a stronger intermolecular interaction and therefore, more energy is needed to break it pushes the molecules to the gas phase. The tertiary amine, on the other hand, has no hydrogens and boils at a lot lower temperature.
How does hydrogen bonding affect physical properties?
As expected, hydrogen bonding affects the physical properties which we can see, for example, by comparing the boiling point of ethanol shown above and dimethyl ether. These two are constitutional isomers meaning they have the same chemical formula and therefore, molecular mass.
What type of interaction occurs when a hydrogen atom is bonded to an electronegative atom?
You can check the previous post for more details about the hydrogen bonding. In short, it is another type of intermolecular electrostatic interaction that occurs between a hydrogen atom bonded to an electronegative atom such as O, N, or F, is attracted to a lone pair of electrons on an atom in another molecule.
How do molecules turn into gas?
Now, to turn into the gas phase, the molecules should overcome the intermolecular interactions and escape the liquid surface , and the stronger these interactions, the harder it is for the molecules to overcome those.
What Are Boiling Points of Compounds?
Any liquid is susceptible to being heated. When the temperature is gradually increased, it reaches a point ay which the great vapor pressure that forms bubbles inside the liquid. At that moment, the liquid begins to boil and transforms into a gaseous state. This temperature is called the boiling point.
What Determines Boiling Point?
The boiling point of a compound is influenced by several factors. The first of these is pressure. At lower pressure, a lower temperature is needed for a compound to start boiling. In turn, when the pressure is higher, a higher temperature is needed to boil the compound. Height is also related to the boiling point of compounds.
Determining Which Compound Has the Highest Boiling Point?
Intermolecular forces attract or repel two or more molecules. They determine the properties of compounds, such as the boiling point. There are four intermolecular forces:
What do you mean by boiling point?
Boiling point of a substance or compound is that value of temperature at which the vapour pressure of the liquid/substance becomes equal in magnitude to the value of ambient pressure.
How to find highest boiling point of a compound?
The boiling point of compound depends on many factors. If we want to find the compound with higher boiling point then we refer the following factors-
How to find lowest boiling point of a compound?
We have already discussed on how to determine whether the boiling point will be high or not. We can use the same factors to find out whether the boiling point will be low or not.
How to find the boiling point of a chemical compound?
Boiling point of any chemical compound can be found using following steps. We shall consider an example in which we take a solution. The solution consists of solute and solvent. The equation that we will use is called as Clausius Clapeyron equation.
How to find the boiling point of an organic compound?
We can find the boiling point of an organic compound by performing a simple experiment. The experiment is discussed in the section given below. We shall directly jump to the procedure part of the experiment.
Which compound has the highest boiling point?
Ethanol or Ethyl alcohol has the highest boiling point. This is due to the high impact of van der waal force and dipole dipole interactions. These forces are a part of strong attractive forces between the molecules.
Which organic compound has the highest boiling point?
When we talk about organic compounds, Propan-1-ol or Propanol has the highest boiling point. This is explained by the strong H bonds and OH group present in the compound.