
What is the Ostwald process in chemistry?
The Ostwald process is a chemical process that in two stages, converts ammonia to nitric acid (also known as HNO3). In the process for step 1, ammonia is oxidized to form nitric oxide and also nitrogen dioxide. Then in step 2, the nitrogen dioxide that was formed is absorbed in water. Furthermore, why was the Ostwald process important?
What is the Ostwald process for nitric acid?
The Ostwald process is a chemical process for making nitric acid (HNO 3 ). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production.
How do you change the equilibrium in the Ostwald process?
The Ostwald Process. By decreasing the temperature, because this reaction is exothermic, the equilibrium will shift to the left away from the added energy. So for favorable conditions, we would want to decrease the temperature. By increasing the concentration, the equilibrium will shift away from the added product or reactant.
What is a catalyst in chemistry?
A catalyst is a substance that increases the rate of a chemical reaction. A catalyst that is used in this reaction is a platinum gauze, and sometimes even a copper wire/rod can serve as a catalyst for this process (Ostwald's Method-Industrial Preparation, 2016).
What is the Ostwald process?
What is stage 2 of nitric oxide?
About this website
Which catalyst is used in the manufacture of HNO3 by Ostwald process?
Ostwal's process is a chemical process for the manifacture of nitric acid in large scale.In the ostwalds process, during the preparation of HNO3, which can use for fertilisers, vanadium pentaoxide (V2O5) is used as catalyst.
Why is platinum used as a catalyst in the Ostwald process?
In general, the catalytic oxidation of ammonia uses a metal catalyst, such as platinum, copper, or nickel. This metal catalyst is heated, so that ammonia can reduce it. Oxygen added to the system can then oxidize the ammonia, forming nitric oxide. When nitric oxide is mixed with water, nitric acid is formed.
Is HNO3 a catalyst?
Nitric acid, HNO3, was known to the alchemists of the 8th century as “aqua fortis” (strong... The principal method of manufacture of nitric acid is the catalytic oxidation of ammonia.
What is the catalyst used in the contact process?
vanadium oxideThe contact process is a present method of producing concentrated sulphuric acid which is required for industrial use. The catalyst used during the process is vanadium oxide.
Where is platinum used as a catalyst?
Platinum catalysts are used in the production of silicones, high-octane gas, and petrochemical feedstocks, which are used to make plastics, synthetic rubber, and polyester fibers. Platinum and palladium are widely used in applications requiring stability at high temperatures such as jet engine and missile parts.
Why is platinum used as a catalyst in nitric acid?
In order to convert as much of the ammonia as possible, the process is carried out under pressure with a platinum-rhodium catalyst. The nitric oxide goes through another oxidation process to create nitric dioxide, which is mixed with water to create nitric acid.
What catalyst is used in nitric ammonia oxidation process?
platinum–rhodium catalyst(3) In this process, ammonia is combusted (oxidized) in air, to nitric oxide (NO). This highly exothermic reaction is carried out over a highly selective platinum–rhodium catalyst.
What called H2SO4?
Sulfuric Acid, H2SO4, is a strong mineral acid that is soluble in water. It is a colorless oily liquid that is extremely corrosive and sometimes called oil of vitriol. sulfuric acid - chemical formula C2H4 is a mineral acid that is soluble in water.
What is catalytic oxidation of ammonia?
Ammonia when reacts with oxygen in presence of platinum as catalyst at 800°C ammonia is oxidised to nitric oxide NO. This is called catalytic oxidation of ammonia.
Why is vanadium oxide used as a catalyst?
Vanadium(V) oxide as a Catalyst This is a good example of the ability of transition metals and their compounds to act as catalysts because of their ability to change their oxidation state (oxidation number).
Which catalyst is used in hydrogenation of oil?
Nickel catalystNickel catalyst is used in commercial hydrogenation of edible oils. Other catalysts, such as platinum, palladium, copper, etc., have also been applied in hydrogenation applications.
What is a catalyst in chemistry?
A catalyst is a substance that speeds up a chemical reaction, or lowers the temperature or pressure needed to start one, without itself being consumed during the reaction.
What is the Ostwald process?
Ostwald process is the process used for the manufacture of nitric acid.
Which transition metal complex is used as a catalyst in Ostwald’s process?
Vanadium Pentoxide is used as a catalyst in Ostwald’s process.
Is Ostwald’s process exothermic or endothermic?
Ostwald’s process is exothermic. Heat is released during the reaction.
1. What catalyst is used in the Ostwald Process?
Ammonia is converted to nitric acid in two stages. It is oxidized by heating with oxygen in the presence of a catalyst which is platinum that has t...
2. Explain the Ostwald Process.
The volume of dry ammonia and dry air in the ratio 10:1 is mixed, compressed and then passed into a platinum gauze which is the catalyst to the rea...
3. Why is the Ostwald Process considered important?
The Ostwald Process was initially important because the Germans needed it during the first world war for the purposes of explosives since it can al...
4. What is the formula of Nitric Acid?
In this, the nitrogen atom is bonded by an equivalent bond to a hydroxyl group and the remaining oxygen atoms. It serves a role as a reagent and pr...
5. What is the principle behind the Ostwald Process?
The conversion of ammonia to nitric acid is a result of oxidation. This oxidation results in nitric oxide. Furthermore, nitric oxide can be oxidize...
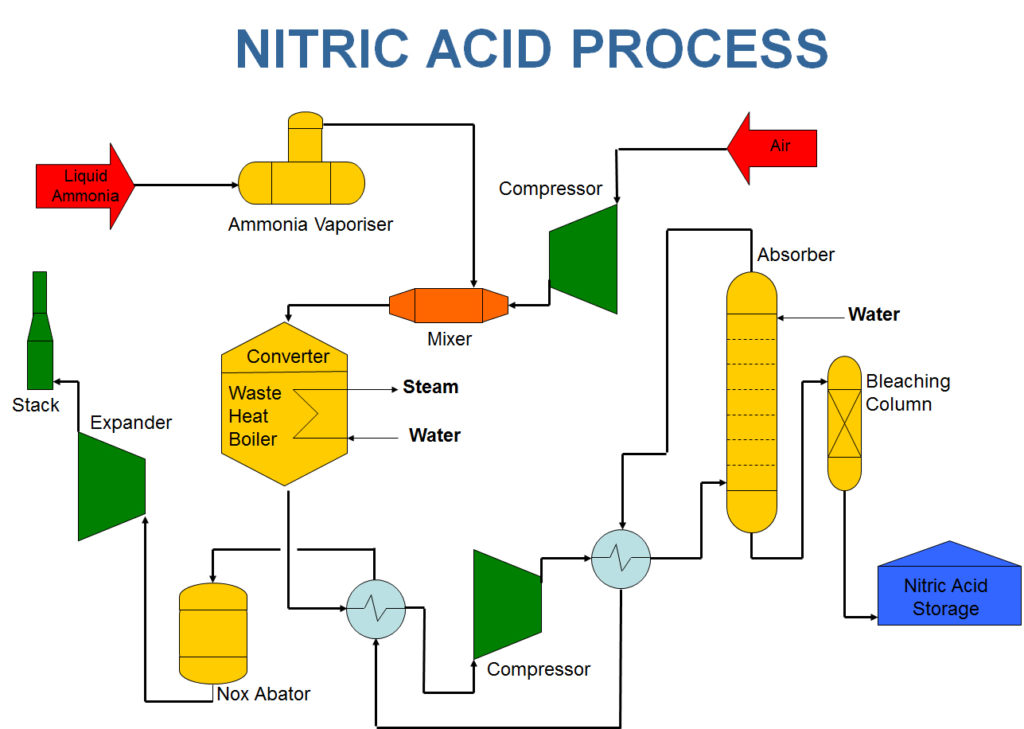
Step 1 – Catalytic Oxidation Reaction
The main goal in this process is the conversion of ammonia into nitric acid. The process begins in a catalyst chamber where one ammonia part and eight oxygen parts are introduced by volume. The chamber temperature is around 600 o C. This chamber uses a catalyst-like platinum gauze or copper and nickel can also be used.
Step 2 – Absorption of No 2 (Formation of HNO 3)
In a special absorption tower containing water, the nitrogen dioxide from the secondary oxidation chamber is introduced. NO 2 gas is passed through a tower where it absorbs the water. Nitric acid is then obtained through this process.
Why is the Ostwald process important?
Ans: The ostwald's process is considered as a very important process because it provides a key ingredient for many of the fertilizers, nitrate ions and these fertilizers improve the plants growth.
Who invented fertilizer?
Wilhelm Ostwald developed this process, and inj 1902 he patented it. This process is a mainstay of the modern chemical industry, and it also provides the main raw material for the most common type of fertilizer production in the world.
Why don't industrial plants use Ostwald?
Because of it's reaction when combined with organic compounds, most industrial probably don't use the most favorable conditions during the Ostwald process while creating because it will produce an unsafe concentration and corrosive tendencies within the nitric acid.
When was the Ostwald patented?
It was created in 1902, patented in 1902, he then later was awarded the Nobel-peace prize for his work in 1909. Wilhelm Ostwald was born in Riga, Russian Empire to mother Elisabeth Leuckel and father Gottfried Wilhelm Ostwald (www.nobelprize.org, 1966). This process was and still is a very important process because it is any easy way ...
Why is Wilhelm's process still being used today?
So the process the Wilhelm created is still being used today because it is reliable and the easiest way to create nitric acid for the high demand it is needed for. In this step, ammonia is heated with oxygen. This yields nitric oxide (NO) and water as products.
What is the nitrogen dioxide that is formed in step 2?
Then in step 2, the nitrogen dioxide that was formed is absorbed in water. This in-turn forms nitric acid (www.pem-news.de, n.d.). The Ostwald process has many well-known uses in both the industrial and health field. Through the Ostwald process, nitric acid is commonly used in fertilizers and pharmaceuticals, and because ...
Is nitric acid a catalyst?
This would be a safety hazard, which is actually why nitric acid is used in explosives. The catalyst that is used for this reaction is a platinum gauze. It would be heated, however sometimes in substitute..a copper wire/rod can serve as a proper catalyst for this process (www.digipac.ca, n.d.).
What is the Ostwald process?
The Ostwald process is a chemical process used for making nitric acid (HNO 3 ). Wilhelm Ostwald developed the process, and he patented it in 1902. The Ostwald process is a mainstay of the modern chemical industry, and it provides the main raw material for the most common type of fertilizer production. Historically and practically, the Ostwald ...
What is stage 2 of nitric oxide?
Stage two encompasses two reactions and is carried out in an absorption apparatus containing water. Initially nitric oxide is oxid ized again to yield nitrogen dioxide (nitrogen (IV) oxide). This gas is then readily absorbed by the water, yielding the desired product (nitric acid, albeit in a dilute form), while reducing a portion ...
