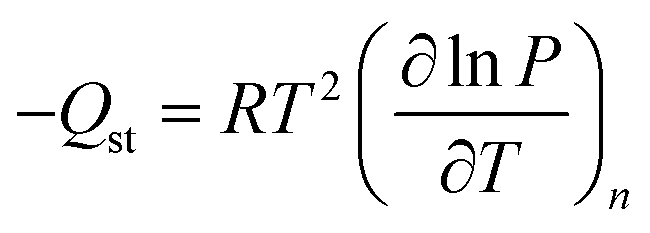
What is the importance of the Clapeyron equation
Clausius–Clapeyron relation
The Clausius–Clapeyron relation, named after Rudolf Clausius and Benoît Paul Émile Clapeyron, is a way of characterizing a discontinuous phase transition between two phases of matter of a single constituent.
What is the Clausius Clapeyron equation thermodynamics?
The clausius clapeyron equation thermodynamics is as follow, ln (P 2 P 1) = Δ H v a p R (1 T 1 - 1 T 2) To determine the ranges of hydrate stability, the Clausius Clapeyron equation can be applied to a hydrating system and used to estimate the equilibrium water behaviour for a hydrate pair occurring in equilibrium at various temperatures.
What is the Clausius equation?
The Clausius equation will describe this action. The temperature of a system determines the balance between a liquid and its vapour; an increase in temperature induces a subsequent rise in the vapour pressure of the liquid.
What is Clausius-Clapeyron?
Clausius-Clapeyron is derived basically from first and second law of thermodynamics, a derivation can be found here: 8.4 The Clausius-Clapeyron Equation. It basically tells you the relation between the directly measurable quantities like pressure and latent heat during either melting or vaporization or in general a phase change.
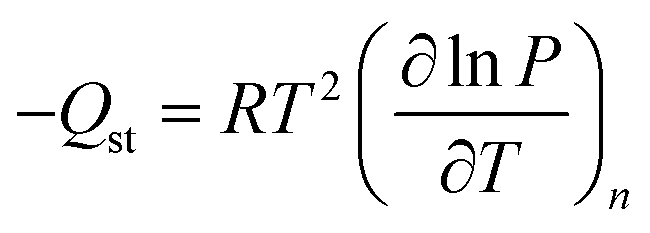
What is the Clausius-Clapeyron relation and why is it important for climate?
The Clausius-Clapeyron relationship predicts an increase in the water holding capacity of air (the saturation water vapor pressure) of approximately 7% per degree Celsius rise in temperature2.
What is Clausius-Clapeyron equation in physics?
The left hand side is the rate of increase of vapour pressure with temperature, while S2 − S1 is equal to L/T, where L is the specific latent heat of vaporization. Thus we arrive at the Clausius-Clapeyron equation: dPdT=LT(V2−VL).
What approximations are involved in the clapeyron Clausius equation?
In this case, ΔV can be approximated as the molar volume of the vapor Vvap. This is the integrated form of the Clausius-Clapeyron equation. If the vapor pressure P1 is known at boiling point temperature T1, this equation can be used to estimate the boiling point temperature T2 at another pressure P2.
Is the Clausius-Clapeyron equation accurate?
This is never really true, but changes in these. H's are very small at low and moderate pressures. As one approaches the critical point, this assumption will fail completely. The vapor is assumed to be an ideal gas.
Who made the Clausius-Clapeyron equation?
atmospheric water vapour … physical relationship known as the Clausius-Clapeyron equation, named for 19th-century German physicist Rudolf Clausius and 19th-century French engineer Émile Clapeyron.
When can the Clausius-Clapeyron equation be used?
The equation describes the phase transition between two phases of matter that have the same composition. Thus, the Clausius-Clapeyron equation can be used to estimate vapor pressure as a function of temperature or to find the heat of the phase transition from the vapor pressures at two temperatures.
What is Clausius-Clapeyron?
It basically tells you the relation between the directly measurable quantities like pressure and latent heat during either melting or vaporization or in general a phase change. ...
Which equation gives the relationship between P and T?
The Clausius-Clapeyron equation gives the relationship between P and T along the phase boundaries such as vapour to solid and vapour to liquid, whereas the Antoine's equation specifically describes the relation between P and T along the vapour-liquid phase boundary. Therefore, Clausius-Clapeyron e quation is more general.
What is the cyclic integral of the change in entropy of the reversible system?
Clausius inequality states that cyclic integral of the the change in entropy of the reversible system is zero.. and for irreversible cycle its value is must less than zero.. The entropy difference between any two states of a system the can be evaluated by the integral of its initial state and final state.
What is the plot between lnp and (1/T)?
As you can see, a plot between lnp and (1/T) would be a straight line and hence only one point at phase change (you can have latent heat only at phase change) is required to plot entire pressure-temperature phase diagram. It basically tells you the relation between the directly measurable quantities like pressure and latent heat during ...
Is entropy a state function?
Since entropy is a state function, the entropy change of the system for an irreversible path is the same as for a reversible path between the same two states. For the reversible process the universe is brought back to its initial state without changing it's properties. So, the entropy generation.
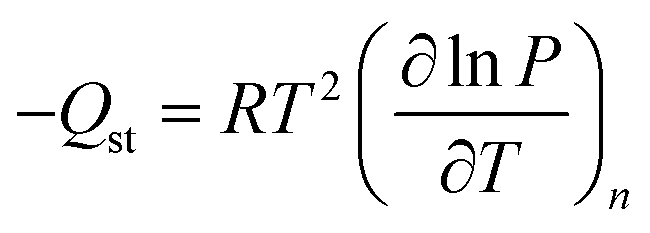
The Clausius Clapeyron Equation Derivation
- The Clausius Clapeyron equation predicts the rate at which vapour pressure increases per unit increase in temperature for a substance's vapour pressure (P) and temperature (T). dlnpdT=ΔHvapRT2 The molar enthalpy of vaporisation of the liquid, the ideal gas constant, and the temperature of the system determines the rate at which the natural logarithm of the vapour pressure of a liquid varies with temperature, according to this equation. If Hv…
Potential Uses of Clausius Clapeyron Equation
- The Clausius clapeyron equation has many Potential uses. Some examples are: 1. We can use thermodynamic data to calculate the slope of a metamorphic reaction to see whether it could be used as a geothermometer or geobarometer. A geobarometer could be a reaction with a shallow dP/dT slope because it is more sensitive to pressure changes. A reaction with a steep (nearly vertical) slope is vulnerable to temperature and maybe a geoth…
Clausius Clapeyron Equation Derivation in Thermodynamics
- The temperature of the system affects the balance between water and water vapour. The saturation pressure of water vapour rises as the temperature rises. The Clausius Clapeyron equation calculates the rate of increase in vapour pressure per unit increase in temperature. Let T be the temperature and p be the saturation vapour pressure. The Clausius Clapeyron equation for liquid-vapour equilibrium is then used. dpdT=L(T(Vv−Vl)) where L …
Applications of The Clausius Clapeyron Equation
- The equation can be used for the following: 1. To determine the slope of a metamorphic reaction from thermodynamic data. This is used to find out if it could be a potential geothermometer or geobarometer. 2. The equation can help us determine thermodynamic values for reactions or phases. 3. When we perform a Schreinemakers analysis of an invariant ...