What is the concentration of potassium in the intracellular compartment?
The vast majority of potassium is in the intracellular compartment with a small amount in the extracellular space. Normal serum potassium is 3.5 to 5.5 mEq/L; however, plasma potassium is 0.5 mEq/L lower. While total body potassium is lower in females and in older patients, serum potassium concentration is independent of sex and age.
What is the normal range of potassium in the body?
Potassium is an intracellular cation with 98% of body potassium located intracellularly. Cell potassium concentration is around 140 mmol/L, whereas the normal range for plasma potassium varies between 3.2 and 6.2 mmol/L depending on age. Reference ranges vary with the method used and age of the child.
What is the molecular weight of potassium in the body?
Definition. Potassium, a metallic inorganic ion with atomic weight of 39, is the most abundant cation in the body. The vast majority of potassium is in the intracellular compartment with a small amount in the extracellular space. Normal serum potassium is 3.5 to 5.5 mEq/L; however, plasma potassium is 0.5 mEq/L lower.
What is the difference between extracellular and intracellular potassium?
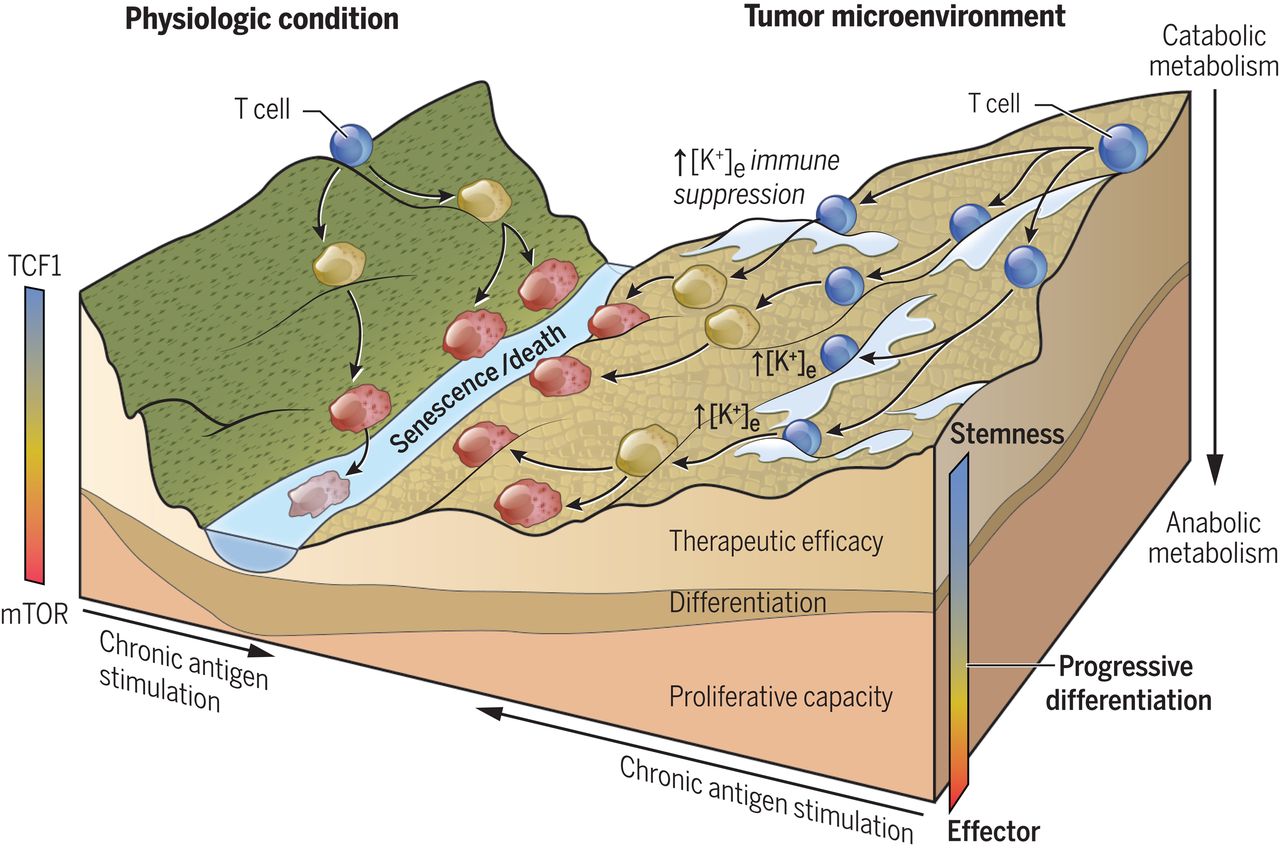
Most potassium resides intracellularly, and a small amount is in extracellular fluid [ 2-4 ]. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the sodium-potassium (Na+/K+) ATPase transporter [ 4 ].
See more
How much potassium is in a cell?
Roughly 98% of the potassium in your body is found in your cells. Of this, 80% is found in your muscle cells, while the other 20% can be found in your bones, liver and red blood cells ( 6 ).
What is the concentration of potassium?
Normal serum potassium is 3.5 to 5.5 mEq/L; however, plasma potassium is 0.5 mEq/L lower. While total body potassium is lower in females and in older patients, serum potassium concentration is independent of sex and age.
What is the intracellular concentration of potassium?
Abstract. Potassium is the most abundant exchangeable cation in the body. It exists predominantly in the intracellular fluid at concentrations of 140 to 150 meq/liter and in the extracellular fluid at concentrations of 3.5 to 5 meq/liter.
Why is potassium higher in cells?
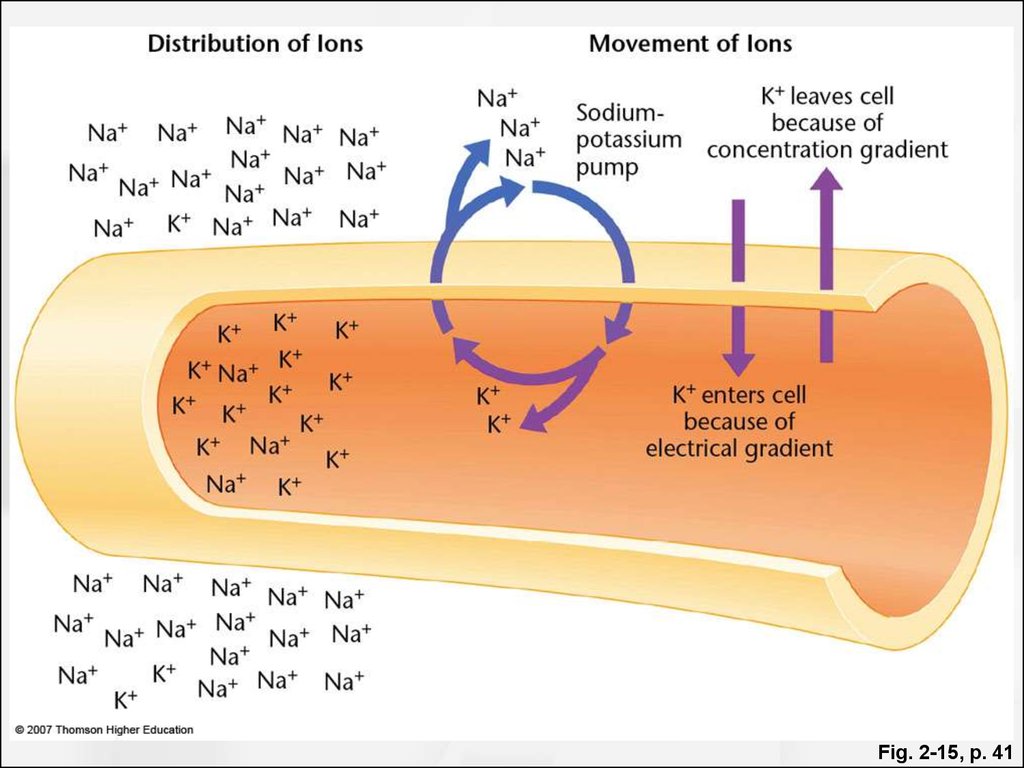
The sodium and chloride ion concentrations are lower inside the cell than outside, and the potassium concentration is greater inside the cell. These concentration differences for sodium and potassium are due to the action of a membrane active transport system which pumps sodium out of the cell and potassium into it. A.
How do you measure potassium concentration?
Potassium is usually measured using an Ion-selective electrode (ISE), which converts the activity (or effective concentration) of the ion dissolved in solution into an electric potential measured by a voltmeter. Both plasma and serum can be used to measure potassium.
Is potassium in or out of the cell?
Most of the body's potassium ions stay inside the cells. In fact, potassium is the most abundant positively charged ion found inside the cell walls. The remaining amount of potassium can be found in the bloodstream.
Is potassium higher intracellular or extracellular?
Background: Potassium (K+) is the major intracellular cation, with 98% of the total pool being located in the cells at a concentration of 140-150 mmol/l, and only 2% in the extracellular fluid, where it ranges between 3.5 and 5 mmol/l.
What is the intracellular concentration of sodium?
Intracellular sodium concentration is 12 mEq/L (12 mmol/L). Extracellular sodium concentration averages 140 mEq/L (140 mmol/L).
What is the highest potassium level recorded?
The highest recorded levels of potassium were 9 mmol/L [25] and 6.4 mmol/L in an adult who was successfully resuscitated from hypothermic CA and an avalanche victim, respectively [26]. A potassium level < 12 mmol/L in a hypothermic CA patient usually indicates ECLS rewarming [1].
How much potassium is in the human body?
The total amount of potassium in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 mmol = 1 milliequivalent [mEq] or 39.1 mg potassium) [3]. Most potassium resides intracellularly, and a small amount is in extracellular fluid [2-4].
What happens when potassium is high?
Hyperkalemia occurs when potassium levels in your blood get too high. Potassium is an essential nutrient found in foods. This nutrient helps your nerves and muscles function. But too much potassium in your blood can damage your heart and cause a heart attack.
Do white blood cells contain potassium?
These calculations indicate that 15,000 white cells contain as much potassium as 1,000,000 platelets. Although the mechanism of the potassium release is not fully understood the present study and previous publications suggest some of the factors involved.
What is the concentration of K+ in molarity?
73×10−3⋅g329.24⋅g⋅mol−11⋅L=2.21×10−5⋅mol⋅L−1 with respect to K3[Fe(C≡N)6] . Since that are 3 moles of potassium ion per mole of salt, [K+]=6.7×10−5⋅mol⋅L−1 .
How much potassium is in a unit of blood?
1 Accordingly, the total amount of extracellular potassium in a unit of whole blood stored for 35 days is about 8.2 mmol and that of red cell concentrate is 5.5 mmol.
What is the normal level of potassium in urine?
For adults, normal urine potassium values are generally 20 mEq/L in a random urine sample and 25 to 125 mEq per day in a 24 hour collection. Lower or higher urinary level may occur depending on the amount of potassium in your diet and the amount of potassium in your body.
What is normal sodium and potassium levels?
The mean baseline serum potassium and sodium levels were 4.47 ± 0.35 mEq/L and 142.67 ± 2.64 mEq/L, respectively. A total of 3.8% of participants had serum potassium level above the normal range (normal: 3.5–5.1 mEq/L), while 0.8% and 6.7% had lower and higher serum sodium level, respectively (normal: 136–146 mEq/L).
What is the normal potassium level?
Normal serum concentrations of potassium range from about 3.6 to 5.0 mmol/L and are regulated by a variety of mechanisms [ 3, 7 ]. Diarrhea, vomiting, kidney disease, use of certain medications, and other conditions that alter potassium excretion or cause transcellular potassium shifts can cause hypokalemia (serum levels below 3.6 mmol/L) or hyperkalemia (serum levels above 5.0 mmol/L) [ 3, 5, 7, 8 ]. Otherwise, in healthy individuals with normal kidney function, abnormally low or high blood levels of potassium are rare.
What is the cause of hypokalemia?
Severe potassium deficiency can cause hypokalemia, (serum potassium level less than about 3.6 mmol/L) [ 3, 7, 8 ]. Hypokalemia affects up to 21% of hospitalized patients, usually because of the use of diuretics and other medications [ 29, 30 ], but it is rare among healthy people with normal kidney function.
How much potassium is in the body?
The total amount of potassium in the adult body is about 45 millimole (mmol)/kg body weight (about 140 g for a 175 pound adult; 1 mmol = 1 milliequivalent [mEq] or 39.1 mg potassium) [ 3 ]. Most potassium resides intracellularly, and a small amount is in extracellular fluid [ 2-4 ]. The intracellular concentration of potassium is about 30 times higher than the extracellular concentration, and this difference forms a transmembrane electrochemical gradient that is maintained via the sodium-potassium (Na+/K+) ATPase transporter [ 4 ]. In addition to maintaining cellular tonicity, this gradient is required for proper nerve transmission, muscle contraction, and kidney function.
How much potassium is in a multivitamin?
Not all multivitamin/mineral supplements contain potassium, but those that do typically provide about 80 mg potassium [ 18 ]. Potassium-only supplements are also available, and most contain up to 99 mg potassium. Information on many dietary supplements that contain potassium is available in the Dietary Supplement Label Database from the National Institutes of Health, which contains label information from tens of thousands of dietary supplement products on the market.
How much potassium is absorbed by humans?
A 2016 dose-response trial found that humans absorb about 94% of potassium gluconate in supplements, and this absorption rate is similar to that of potassium from potatoes [ 24 ].
What is the form of potassium in fruits and vegetables?
The forms of potassium in fruits and vegetables include potassium phosphate, sulfate, citrate, and others, but not potassium chloride (the form used in salt substitutes and some dietary supplements; see supplements section below) [ 16 ]. Selected food sources of potassium are listed in Table 2.
What is the RDA for nutrition?
These values, which vary by age and sex, include: Recommended Dietary Allowance (RDA): Average daily level of intake sufficient to meet the nutrient requirements of nearly all (97%–98%) healthy individuals; often used to plan nutritionally adequate diets for individuals.
How do ion channels work?
Ion channels serve as passive barriers that can be opened or closed in response to environmental cues such as voltage across the membrane, the concentration of ligands or membrane tension. Pumps, by way of contrast, use energy in the form of protons or ATP in order to pump charged species against their concentration gradient. The differences in concentration mediated by these membrane machines can often be several orders of magnitude and in the extreme case of calcium ions correspond to a 10,000-fold greater concentration of ions outside of the cell than inside as shown in Table 1. The dominant players in terms of abundance inside the cell are potassium (K + ), chloride (Cl –) and magnesium (Mg 2+) (though the latter is mostly bound to ATP, ribosomes and other macromolecules and metabolites such that its free concentration is orders of magnitude lower). Table 1 shows some typical ionic concentrations in bacteria, yeast and mammalian cells. Some ion concentrations are regulated tightly, particularly toxic metal ions that are also essential for certain processes, but also regulation of K + by osmolarity, which is essential for growth. Other ions are less tightly regulated, Na + being one such example. One of the provocative observations that emerges from this table is that positive ions are much more abundant than negative ions. What is the origin of such an electric imbalance in the simple ions? Many of the metabolites and macromolecules of the cell are negatively charged. This negative charge is conferred by phosphate in small metabolites and DNA and by carboxylic groups on the acidic amino acids, such as the most abundant free metabolite, glutamate. Much more on these cellular players can be found in the vignette on “What are the concentrations of free metabolites in cells?”.
How is potassium in equilibrium?
Potassium is usually close to equilibrium in animal and plant cells. Given that its concentration inside the cell is about 10 to 30 fold higher than outside the cell, how can it be in equilibrium? Assume we start with this concentration difference across the membrane, and with no electric potential difference (there are counter ions on each side of the membrane to balance the initial charges and they cannot move). As the potassium ions diffuse down their concentration gradient, from the inside to the outside, they quickly create an electric potential difference due to their positive net charge (the net charge movement is miniscule compared to the ion concentrations on the two sides of the membrane as discussed in the vignette on “What is the electric potential difference across membranes?”). The potential difference will increase until its effect will exactly balance the diffusive flux and this is when equilibrium will be reached. This type of equilibrium is known as electrochemical equilibrium. Indeed from the equilibrium distribution we can infer that the cell has a negative electric potential inside and by how much. The direction of the voltage difference across the cell membrane is indeed from positive outside to negative inside as can be naively expected from pumping of protons out of the cell, and as discussed in quantitative terms in the vignette on “ What is the electric potential difference across membranes?”.
What is negatively charged in a cell?
Many of the metabolites and macromolecules of the cell are negatively charged . This negative charge is conferred by phosphate in small metabolites and DNA and by carboxylic groups on the acidic amino acids, such as the most abundant free metabolite, glutamate. Much more on these cellular players can be found in the vignette on “What are ...
What are the roles of ions in cells?
Ions have a huge variety of roles in cells. Several of our favorites include the role of ions in electrical communication (Na +, K +, Ca 2+ ), as cofactors in dictating protein function with entire classes of metalloproteins (constituting by some estimates at least ¼ of all proteins) in processes ranging from photosynthesis to human respiration ...
Why is Na+ hard to measure?
Na+ concentrations are especially hard to measure due to trapping and sticking of ions to cells. Most Mg2+ ions are bound to ATP and other cellular components.
What are the dominant players in terms of abundance inside the cell?
The dominant players in terms of abundance inside the cell are potassium (K + ), chloride (Cl –) and magnesium (Mg 2+) (though the latter is mostly bound to ATP, ribosomes and other macromolecules and metabolites such that its free concentration is orders of magnitude lower). Table 1 shows some typical ionic concentrations in bacteria, ...
What is the y axis?
The y-axis is in units of ionic concentration called Eq for “equivalents”, which are equal to the ion concentration multiplied by its absolute charge. These units make it easy to see that the total amount of positive and negative charge is equal in each compartment, in line with the principle of electro-neutrality.
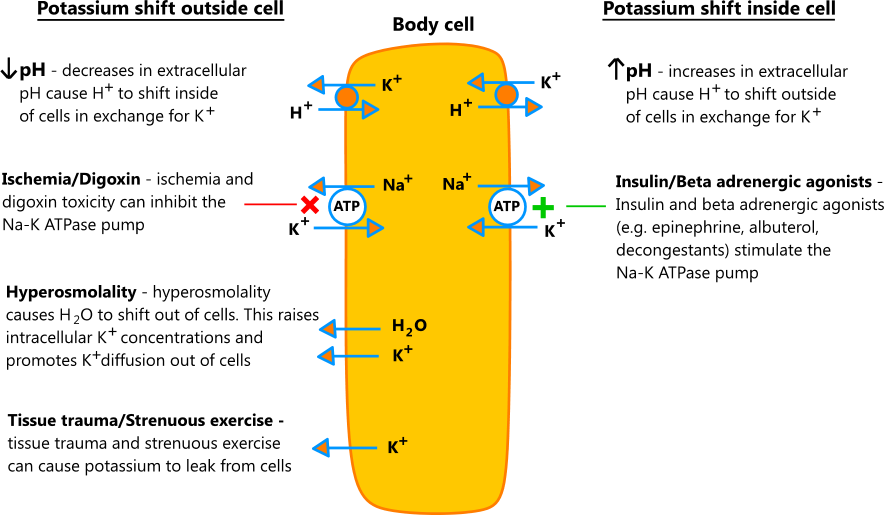
What happens to potassium in acidemia?
The reverse occurs during acidemia with a shift of potassium out of the cell. A sudden increase in plasma osmolality will shift water out of the cell and drag some potassium with the water. This will result in a significant rise in serum potassium. Long-term potassium homeostasis is maintained by the kidney.
How much potassium is in the body?
Total body potassium is approximately 55 mEq/kg body weight. Of this amount, 98% is in the intracellular compartment (primarily in the muscle, skin, subcutaneous tissue, and red blood cells) and 2% is in the extracellular compartment. Therefore in a 70-kg man with total body potassium of 3750 mEq, approximately 3675 mEq is in ...
How does acid-base balance affect potassium?
Acid–base balance affects serum potassium by the exchange of hydrogen ions for potassium across the cell membrane. A rise in the serum pH (decrease in H+concentration) will result in a shift of H+out of the cell and potassium into the cell. The reverse occurs during acidemia with a shift of potassium out of the cell.
What is the normal potassium level?
Normal serum potassium is 3.5 to 5.5 mEq/L; however, plasma potassium is 0.5 mEq/L lower. While total body potassium is lower in females and in older patients, serum potassium concentration is independent of sex and age. Technique. Serum potassium is measured by the use of a flame photometer or ion-selective electrode.
How is potassium maintained in the kidney?
Potassium is freely filtered and then 90% reabsorbed in the tubular segments proximal to the distal convoluted tubule. This is independent of potassium intake and final urinary potassium. The majority of the potassium excreted is secreted by specialized cells in the distal convoluted tubule and/or cortical and outer medullary collecting ducts (Figure 195.2). Under severe potassium deprivation, the collecting duct can reabsorb potassium; however, urinary potassium never approaches zero, and therefore there is an obligatory potassium loss of 10 to 15 mEq/day. The factors influencing potassium secretion (and therefore excretion) are:
What is the intracellular potassium concentration?
The intracellular potassium concentration is on average 150 mEq/L. The ratio of intracellular to extracellular potassium (KI:Ke) is the major determinant of the resting membrane potential and plays a crucial role in the normal functioning of all cells, specially those with inherent excitability.
How to measure potassium?
Serum potassium is measured by the use of a flame photometer or ion-selective electrode. The procedure is rapid, simple, and reproducible. In interpreting serum potassium, it should be kept in mind that because the intracellular potassium concentration is approximately fortyfold greater than the extracellular concentration, any maneuver that would result in the release of a small amount of intracellular potassium will erroneously raise serum potassium. These include: (1) tight tourniquet; (2) vigorous exercise of the extremity during blood drawing; (3) hemolysis due to vigorous shaking of the test tube; (4) thrombocytosis (platelet count greater than 600,000); and (5) leukocytosis (WBC greater than 200,000). In the last two situations, the longer the blood stands, the greater the rise in serum potassium will be.