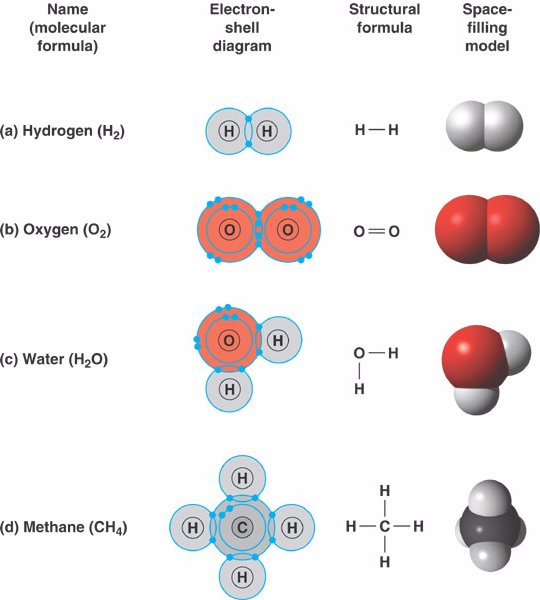
What are 10 examples of covalent compounds?
What are 4 compounds from your everyday life that are covalent?
- Water.
- Sugar.
- Oxygen.
- Carbon Dioxide.
- LPG.
- Vinegar.
- Nail Polish Remover.
- Diamonds.
What are the rules for naming covalent compounds?
Naming Covalent Compounds • When naming Covalent Compounds follow these IUPAC rules: 1. The non-metal closest to the left of the periodic table goes first and keeps its name 2. The second non-metal element is named with the suffix “-ide” 3. “Descriptive Prefixes” are added to the beginnings of the names of both elements. These prefixes are
How to write covalent compound?
- There are no charges involved with covalent compounds.
- Interpret the prefixes to represent the number of atoms or the subscript.
- Do not cross or reduce subscripts (unlike ionic compounds).
- The formula should reflect the name of the compound.
How to name Binary covalent compounds?
There are three types of binary compounds:
- Type I. A metal of fixed charge and a nonmetal;
- Type II.
- Type III.
- The rules for naming binary compounds are then as follows:
- I = 1; II = 2; III = 3; IV = 4; V = 5; VI = 6; VII = 7.
- mono = 1; di = 2; tri = 3; tetra = 4; penta = 5; hexa = 6; hepta = 7; octa = 8.
What is the chemical formula for CO2?
What is the covalent bond?
How to tell if a compound is covalent?
What are the two types of chemical bonds?
What are the subatomic particles that are negatively charged?
Is CO2 ionic or covalent?
Is CO2 a gas?
See 2 more

Is CO2 Ionic or Covalent? - Reference.com
Carbon dioxide, or CO2, forms a covalent bond. Any compound made up of non-metals will form a covalent bond, while compounds made of a metal and non-metal form an ionic bond. A covalent bond takes place when two atoms share electrons, thus binding the two atoms together.
Is CO2 an Ionic Compound -Yes or No - BYJUS
No, CO 2 is not an ionic compound. As per the definition, an ionic compound is a compound that is mostly formed between a metal atom and a non-metal atom. Meanwhile, CO 2 is a compound that is formed between two non-metal atoms (carbon and oxygen) thus giving it a covalent nature. In CO 2 one carbon atom will share its four electrons with two electrons from each of the oxygen atoms.
Is CO (Carbon monoxide) Ionic or Covalent/Molecular? - YouTube
To tell if CO (Carbon monoxide) is ionic or covalent (also called molecular) we look at the Periodic Table that and see that C is a non-metal and O is a non-...
What is the chemical formula for CO2?
CO2 is the chemical formula of the molecular compound of Carbon Dioxide. It is one of the most important compounds and we are familiar with CO2 since childhood. We already know the role of CO2 in respiration. Other than that, we also identify its role in climate change since it is a greenhouse gas.
What is the covalent bond?
Below is the image of the atoms sharing electrons and forming a covalent bond. In covalent bonds, we have the concept of polarity. While ionic bonds already have atoms bearing charges, covalent bonds are said to be polar or nonpolar based on electronegativity differences. So, the main factor determining the nature of covalent bonds is ...
How to tell if a compound is covalent?
A compound is said to be covalent when it has an electronegativity difference between its bonded atoms to be less than 1.8-2. In a CO2 molecule, between C and O, the difference is around 0.89, so we can call this a covalently bonded molecule. Other than this, here in each bond of C and O, the atom of carbon shares four electron pairs ...
What are the two types of chemical bonds?
This chapter deals with various types of chemical bonds. However, in this article, we will restrict our discussion to two major types: Ionic and Covalent.
What are the subatomic particles that are negatively charged?
Atoms have negatively charged subatomic particles called electrons around the nucleus. The electrons present in the outermost shell, also known as valence electrons, have a tendency to take part in the bond formation as a result of which we get several products.
Is CO2 ionic or covalent?
The solid form of CO2 that we use as a refrigerant. Now, you might be wondering about the nature of bonding in CO2 – whether it is an ionic or covalent compound. So, today, in this article, we will discuss Carbon Dioxide – whether it is ionic or covalent in nature. A compound is said to be covalent when it has an electronegativity difference ...
Is CO2 a gas?
CO2 has a molecular weight of about 44.009 g/mol. It is acidic but fairly non-toxic. It bears a colorless and odorless gaseous appearance which is capable of dissolving in water to form carbonic acid H2CO3. We can produce and manufacture this compound via several means.