What is dispersed phase in medical terms?
Medical Definition of dispersed phase. : the phase in a two-phase system that consists of finely divided particles (as colloidal particles), droplets, or bubbles of one substance distributed through another substance. — called also discontinuous phase, internal phase.
What is the difference between the dispersed phase and the dispersion medium?
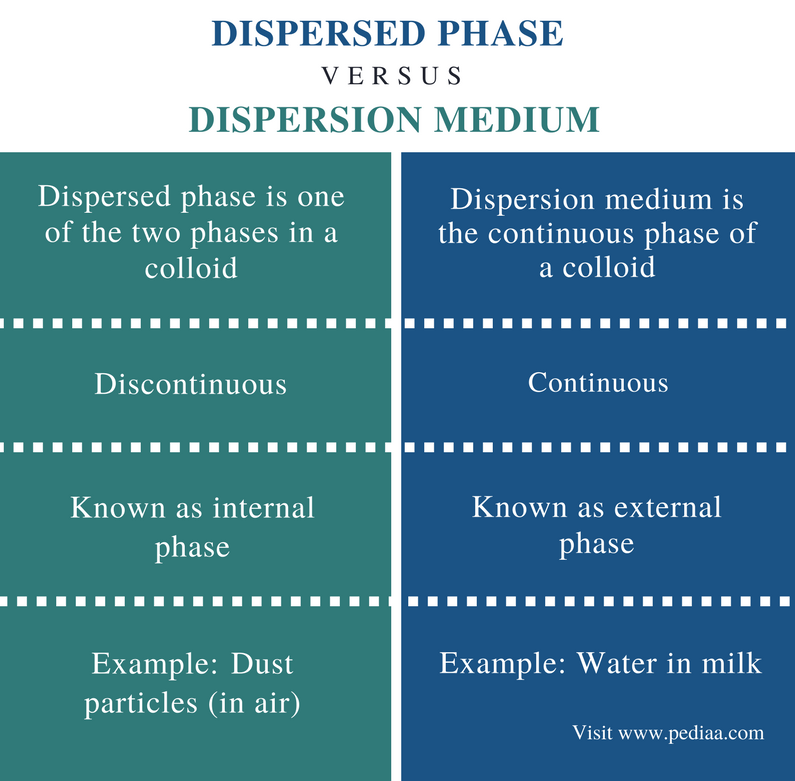
The dispersed phase is known as the internal phase, whereas the dispersion medium is called the external phase. Examples of the dispersed phase include dust in the air, whereas that of dispersion medium includes water in milk. There are two types of dispersion systems, Molecular Dispersions and Coarse Dispersions.
What is dispersed-phase polymerization?
Dispersed-phase polymerization is generally referred to as emulsion or suspension polymerization and is often used for surface imprinting as well.
What is dispersed media?
A dispersed medium consists of two media that do not mix. These two media can be different in properties altogether. Precisely, they can be a liquid, a solid or a gas. There are various everyday life examples of dispersed media. Milk, which we most probably use daily, is made up of oil drops dispersed in water.
.jpg)
What is dispersed phase simple Definition?
What is Dispersed Phase? A liquid that is suspended in the form of fine droplets in a continuous phase. We have classified colloids on the basis of the dispersed phase and the dispersion medium into categories such as solid sol, sol, aerosol, emulsion, gel, foam, etc.
Whats the Definition of dispersed?
1 : to break up in random fashion the crowd dispersed on request. 2a : to become dispersed the particles dispersed throughout the mixture. b : dissipate, vanish the fog dispersed toward morning.
What is the meaning of dispersed phase in chemistry?
Dispersed phase: The phase that is scattered or present in the form of colloidal particles is known as dispersed phase. Dispersion medium: The medium in which the colloidal particles are dispersed is called dispersion medium.
What are the examples of dispersed phase?
The dispersed phase and dispersion medium examples:Dispersed phaseDispersion mediumExamplesSolidGasDustLiquidSolidButter, jellyLiquidLiquidMilkLiquidGasFog, mist, clouds4 more rows
How are seeds dispersed?
Seed dispersal can be accomplished through both abiotic and biotic mechanisms. Abiotic dispersal involves wind and water; biotic dispersal involves autogenic mechanisms, such as explosive fruits, and various animal agents, including insects, fish, reptiles, birds, and mammals.
What does dispersed mean in geography?
A dispersed settlement, also known as a scattered settlement, is one of the main types of settlement patterns used by landscape historians to classify rural settlements found in England and other parts of the world. Typically, there are a number of separate farmsteads scattered throughout the area.
What is continuous phase and dispersed phase?
dispersed phase is an organic material and the continuous phase is. water or an aqueous solution and is termed water/oil (w/o) if the dispersed. phase is water or an aqueous solution and the continuous phase is an. organic liquid (an "oil"). Note 5: A w/o emulsion is sometimes called an inverse emulsion.
What is mean by dispersed phase and dispersed medium?
The phase that is scattered or present in the form of colloidal particles is called the dispersed phase. The medium in which the colloidal particles are dispersed is called the dispersion medium. Example: In a starch solution, starch represents the dispersed phase, while water represents the dispersion medium.
What dispersed particles?
Definitions of dispersed particles. (of colloids) a substance in the colloidal state. synonyms: dispersed phase. type of: form, phase. (physical chemistry) a distinct state of matter in a system; matter that is identical in chemical composition and physical state and separated from other material by the phase boundary.
What are dispersed systems?
The definition of a disperse system is a two-part system made up of microscopic particles and the medium in which they are suspended. An example of a disperse system is a foam such as shaving cream. noun. 1. A two-phase system, such as a colloid, consisting of a disperse phase in a dispersion medium.
What is the difference between dispersed phase and dispersion phase?
The phase that is scattered or present in the form of colloidal particles is called dispersed phase and the medium in which the colloidal particles are dispersed is called the dispersion medium.In a starch solution ,starch represents the dispersed phase while water represents the dispersion medium.
What is a dispersed medium?
A dispersed medium consists of two media that do not mix. More specifically, it contains discrete elements of one medium which are dispersed in a continuous second medium. The two media can be of very different nature. In particular, they can be a gas, a liquid or a solid.
How do you disperse?
3:488:49Various ways of Seed Dispersal | Science | Grade-3,4 | TutWayYouTubeStart of suggested clipEnd of suggested clipSuch seeds are spread or dispersed by wind small. And light seeds fly away with wind to distantMoreSuch seeds are spread or dispersed by wind small. And light seeds fly away with wind to distant places seeds can float on the breeze or alternatively. They can flutter to the ground.
What does dispersed family mean?
dispersed Add to list Share. The adjective dispersed can describe anything that's spread across a distance. You might use the Internet to stay in touch with your dispersed family, who live everywhere from Maine to California.
What is the dispersed phase of dispersion?
The dispersed phase of dispersion is the discontinuous phase that distributes throughout the dispersion medium. It is one of the two stages of a colloid. The dispersion medium is the continuous phase of a colloid, and it distributes throughout the dispersion medium. The dispersed phase is known as the internal phase, whereas the dispersion medium is called the external phase. Examples of the dispersed phase include dust in the air, whereas that of dispersion medium includes water in milk.
How to separate dispersed phase and dispersion medium?
There are various advanced techniques of separation of the dispersed phase and dispersion medium. One such method is micro-filtration. In colloids, both the stages cannot be separated by simple filtration but can be separated using centrifugation. For example, butter is separated from cream by centrifugation. Q3.
How Does Dispersion Occur?
This whole process is aided by molecular diffusion and convection. Through molecular diffusion phenomena, dispersion occurs through different concentrations of the media introduced throughout the bulk medium. The difference in engagement between dispersed material and the bulk medium creates a concentration gradient that drives the medium's dispersion.
What is a Dispersion System and How Does it Work?
The dispersion system has a two-sided system that is made up of microscopic particles along with a medium that helps them to be suspended. The dispersion system works on a basic principle that it separates the collection of particles from each other. Light can be a common example of dispersion. The second commonly used example is a rainbow. Let us know how this works. The speed of the light ray results in light dispersion. Similarly, the dispersion of a rainbow is a spatial disconnection of a single white light into many different lights.
What are the two types of dispersion systems?
There are two types of dispersion systems, Molecular Dispersions and Coarse Dispersions. Molecular dispersions are dedicated solutions to a solute phase in the solvent. The dispersed phase is homogeneously distributed in the dispersion medium. Examples of molecular dispersion are air (consisting of various gasses like nitrogen and oxygen), electrolytes and alloys.
What is the stage of a dispersed medium?
The stage with finely differentiated particles is called the dispersed phase. A dispersed medium consists of two media that do not mix. These two media can be different in properties altogether. Precisely, they can be a liquid, a solid or a gas. There are various everyday life examples of dispersed media.
What is the second type of dispersion medium?
The second type of dispersion medium is of the coarse kind. These are heterogeneous dispersed systems. Fast sedimentation of the dispersed phase is observed owing to the coarse dispersions, mainly due to gravity.
What is Dispersed Phase?
A liquid that is suspended in the form of fine droplets in a continuous phase.
What is dispersion in sedimentation?
A dispersion is a mechanism in which, in a continuous phase of another substance, scattered particles of one material are dispersed. Particle dispersions too broad for sedimentation are commonly referred to as suspensions, while those with smaller particles are referred to as colloids and solutions.
What is a mixture in which very tiny particles of one substance are evenly distributed across another substance?
A mixture in which very tiny particles of one substance are evenly distributed across another substance. In addition, the particles are bigger than the ones in a solution and smaller than the ones in a suspension. Colloids include ink, milk, and fog.
What is a medium that does not combine?
Two media that do not combine consist of a distributed medium. More precisely, it comprises one medium’s isolated elements which are scattered in a second continuous medium. Both media can be very distinct in essence. They may be a gas, a liquid or a solid in particular.
When the dissolution of smaller molecules of a substance or many atoms takes place, they combine to form?
When the dissolution of smaller molecules of a substance or many atoms takes place, they combine to form a species whose size is in the range of colloidal size. The species formed is known as the multimolecular colloid.
What is the medium in which colloidal particles are distributed?
The medium the colloidal particles are distributed in is called the medium of dispersion. Example: Starch represents the dispersed phase in a starch solution, while water is the dispersing medium.
What does "dispersed" mean in science?
Switch to new thesaurus. Noun. 1. dispersed phase - (of colloids) a substance in the colloidal state. dispersed particles. phase, form - (physical chemistry) a distinct state of matter in a system; matter that is identical in chemical composition and physical state and separated from other material by the phase boundary;
What is the first step in the creation of a formulated dispersion?
The first step in the creation of a formulated dispersion is said to involve combining the dispersed phase (solid pigment, solid resins, etc.) in a liquid matrix.
What are the factors that determine the gas barrier performance of a blend?
In terms of morphology, the shape and size of the dispersed phase are vital factors in determining the gas-barrier performances of blends, in addition to the intrinsic barrier properties of each component [17].
What is dispersed phase polymerization?
Dispersed-phase polymerization is generally referred to as emulsion or suspension polymerization and is often used for surface imprinting as well. The different expressions cannot clearly be differentiated since at the beginning of the synthesis an emulsion (two immiscible liquids) and after the synthesis a suspension (liquid and solid phase) are present [22,23]. The high yield of spherical particles and the simple applicability are advantages of this synthesis technique [24].
How to find fluid phase balance?
The fluid phase balance is obtained by subtracting the dispersed phase balance, Eq. (T2.5) from the mixture momentum balance, Eq. (T2.3). This yields
How do droplets break down?
In the absence of interfacial effects accompanying mass transfer, the droplets break down by impact with elements of packing and finally reach an equilibrium size which is independent of the packing size. Conversely, small droplets gradually coalesce until the equilibrium size is attained. Pratt and his coworkers (5,29) showed that the mean droplet size attained in the tower is well represented by:
How are droplets formed in a reaction?
Droplets of one phase are formed upon rapid stirring in the second phase. The spherical droplets thus provide the resulting particle form. The resulting size of the particles depends on the stirring rate and can be controlled easily. Stabilizing agents that are needed to create a stable dispersion can interfere at the interface of the two phases and influence the interactions between template molecule and functional monomer [24].
When did Ugelstad and al. downscale the size of the resulting polymer particles?
Ugelstad et al. [25] were able to downscale the size of the resulting polymer particles to several hundred nanometres via suspension polymerization in 1973 and came to the conclusion that a different amount of detergent has a high impact on the resulting particle size [25].
Is there symmetry between dispersed phase and continuous phase momentum balances?
Note that there is a lack of symmetry between the dispersed phase and the continuous phase momentum balances. Therefore, in applying these equations to describe boiling going from all liquid to all vapor, it is necessary to switch from liquid continuous phase to gas at some appropriate volume fraction of steam. Equations (T2.5) and (T2.6) can also be used to model flow of neutrally buoyant particles.
Can mass transfer be predicted?
Mass transfer. As in the case of spray columns, it is not yet possible to predict mass transfer rates from first principles . In the absence of any reliable correlations, use may be made of typical values of overall (20,31) and film (32,33) coefficients. A comprehensive summary is given in Perry's Chemical Engineers’ Handbook (22).
What is the temperature of the dispersed phase?
The dispersed phase, which has the tan [Delta] temperature peak at about 4 60 [degrees] C consists primarily of resin and a low concentration of low-molecular-weight ends of the rubber.
Why is PPO in the dispersed phase?
PPO is in the dispersed phase of all specimens because of its high viscosity.
phase
1. one of the aspects or stages through which a varying entity may pass.
disperse phase
The state of a substance in a heterogeneous system in which particles are separated from each other, e.g., oil particles in water.
Are we missing a good definition for dispersed phase? Don't keep it to yourself..
The ASL fingerspelling provided here is most commonly used for proper names of people and places; it is also used in some languages for concepts for which no sign is available at that moment.
Definitions & Translations
Get instant definitions for any word that hits you anywhere on the web!