Full Answer
What is difference between hypotonic and hypertonic solution?
The difference between hypertonic and hypotonic solution is mainly due to the factors like:
- Solute concentration
- Solvent concentration
- Effect on a cell
How to make a hypotonic solution?
Making Hydroponic Solution at Home
- Buy the nutrients. You should buy nitrogen, phosphorus, calcium, etc. ...
- Use clean water. You should use filtered water. ...
- Mix the salts with water. You should add the salts slowing into the water. ...
- Add micronutrients. In another container add 1 quart of water and mix 0.25 tsp of boric acid and 0.1 tsp of manganese chloride.
- Adjust the pH level. ...
- Adjust EC level. ...
What does hypotonic solution do?
Hypotonic solution: A solution that contains fewer dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood. Hypotonic solutions are commonly used to give fluids intravenously to hospitalized patients in order to treat or avoid dehydration.
What does hypotonic solution mean?
In these arenas, hypotonic refers to a solution ’s having less osmotic pressure, or concentration, than another solution between a semi-permeable membrane. In more simpler terms, hypotonic can mean a solution that has a lower concentration of solutes than other solutions, made of the same solutes.

What do you mean by hypotonic solution?
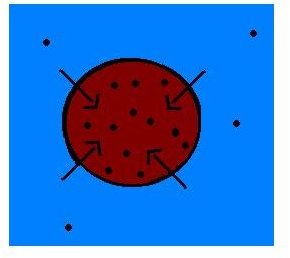
If the solution in the surrounding has a lower solute concentration as compared to the solute concentration inside the cell, then the solution is called hypotonic. Or, if the surrounding solution has high solvent concentration compared to the inside of the cell, then it is hypotonic solution.
What is a hypertonic solution simple definition?
A hypertonic solution has a higher solute content than a cell or another solution. Cells shrink in hypertonic solutions.
What is hypotonic solution and hypertonic?
A hypotonic solution is a type of solution which encompasses anything that contains more water and limited solute compared to the cells. A hypertonic solution is a kind of solution which encompasses anything that contains more electrolytes or molecules than solvent or water.
What is hypertonic solution and example?
Hypertonic solution: A solution that contains more dissolved particles (such as salt and other electrolytes) than is found in normal cells and blood. For example, hypertonic solutions are used for soaking wounds.
Why is hypertonic solution?
A solution will be hypertonic to a cell if its solute concentration is higher than that inside the cell, and the solutes cannot cross the membrane. If a cell is placed in a hypotonic solution, there will be a net flow of water into the cell, and the cell will gain volume.
What is isotonic and hypertonic solution?
In an isotonic solution, no net movement of water will take place. A hypotonic tonic solution is any external solution that has a low solute concentration and high water concentration compared to body fluids. In hypotonic solutions, there is a net movement of water from the solution into the body.
What is hypotonic in biology?
hypotonic: Having a lower osmotic pressure than another; a cell in this environment causes water to enter the cell, causing it to swell. hypertonic: having a greater osmotic pressure than another. isotonic: having the same osmotic pressure.
What are the 3 types of solution?
Hypotonic, isotonic and hypertonic solutions (tonicity).
What is a hypertonic solution in osmosis?
A hypertonic solution is any external solution that has a high solute concentration and low water concentration compared to body fluids. In a hypertonic solution, the net movement of water will be out of the body and into the solution.
How do hypertonic solutions work?
In your body, these solutes are ions like sodium and potassium. Hypertonic solutions cause blood cells to shrivel. Hypotonic solutions can cause the blood cell to burst from the pressure. There are three types of solutions that can occur in your body based on solute concentration: isotonic, hypotonic, and hypertonic.
Does hypertonic shrink or swell?
A hypotonic solution causes a cell to swell, whereas a hypertonic solution causes a cell to shrink.
What is hypertonic saline solution?
Any solution of sodium chloride (NaCl) in water with a concentration of NaCl higher than that found in physiological saline (0.9% w/v).
What is difference between hypotonic and hypertonic solution?
A hypotonic solution is one that has a lower concentration of solute compared to the cell, whereas a hypertonic solution has a higher concentration...
What happens to the cell when placed in a hypotonic solution?
When a cell is placed in a hypotonic solution, water will flow into the cell, causing it to swell. In an animal cell that lacks a cell wall, the c...
What are some examples of hypotonic solutions?
Examples of hypotonic solutions for cells include pure water as well as saline solutions that have less solute than our blood used in medicine, lik...
Why is the cytosol hypotonic?
It means there will be more water per unit of solute in the solution. Since the cell is hypotonic to the surrounding, water will lead to flow out until the cell expands its solute condensate.
What is the difference between isotonic and isotonic solutions?
Isotonic Solution. In the case of an isotonic solution, the extracellular liquid has a similar osmolarity to the cell. Moreover, there will be no net movement of water in or out from the cell. ‘Iso’ means same or similar.
What is the power of an extracellular solution to allow water to move in or out of a cell through o?
The power of an extracellular solution to allow water to move in or out of a cell through osmosis is called ‘tonicity’. A solution’s tonicity relates to its osmolarity. A look at the hypotonic solution will help understand this better.
What is the term for the fluid that has a lower osmolarity than the fluid inside the cell?
If the extracellular fluid has a lower osmolarity than the fluid inside the cell, it’s said to be hypotonic—hypo means less than—to the cell, and the net flow of water will be into the cell.
Which solution has more solute particles per litre?
Whereas, a solution with higher osmolarity has comparatively more solute particles per litre of solution.
Is a solution hypotonic or isotonic?
A solution is not hypotonic, isotonic or hypertonic if there is no solution for comparison. It helps scientists to describe cells. Osmolarity which is the concentration of a liquid in a certain number of solutes per litre of different solutions can tell experts the way in which water gradient and solute gradients can form.
What is an aqueous solution?
aqueous solutionone in which water is used as the solvent.
What is supersaturated solution?
supersaturated solutionan unstable solution containing more of the solute than it can permanently hold.
What is a sclerosing solution?
sclerosing solution one containing an irritant substance (sclerosing agent) that will cause obliteration of a space, as in sclerotherapy. standard solution one that contains in each liter a definitely stated amount of reagent; usually expressed in terms of normality (equivalent weights of solute per liter of solution) or molarity ...
Which solution has the same specific gravity as a standard of reference?
isobaric solutiona solution having the same specific gravity as a standard of reference.
What is a homogeneous mixture?
1.a homogeneous mixture of one or more substances (solutes) dispersed molecularly in a sufficient quantity of dissolving medium (solvent).
What is a Hypotonic Solution?
Imagine you and two other people are waiting for an elevator in the lobby of a building. When the elevator doors open, you see there are 20 people crammed into that tiny space. Who in their right mind would try and fit on that elevator? Not you! There are just too many people in there. The doors close, and you wait for the next one. This is basically what happens when two solutions meet in biology - the two solutions compare their respective crowdedness to each other, and then each solution reacts accordingly. Sometimes, one solution has more 'stuff' crammed into it than the other. We call this stuff solute. The amount of solute in a solution determines how that solution will react when in the presence of another solution.
What is the difference between hypotonic and isotonic?
Second, it may be considered a hypertonic solution, meaning it has more solute and less water than another solution. Third, it may be considered a hypotonic solution, meaning it has less solute and more water than another solution . This is the situation described above - when you were waiting in the lobby, you and the two other people were like solute in a solution, and all that space around was the water. When the elevator doors opened, there were a lot more people in a lot less space in that elevator - a lot more solute in a lot less water. Therefore, your lobby was considered hypotonic when compared to the crammed elevator.
What is the function of solute concentration?
Basically, water wants to dilute that solute. Those solutes are what exert the osmotic pressure. Since solute cannot cross the semipermeable membrane, the amount of solute in a solution determines if water is going to rush across that membrane or not. Therefore, solute concentration controls tonicity.
What is tonicity in cells?
This is where tonicity comes in. Once again, tonicity is a measure of the osmotic pressure gradient of two solutions separated by a semipermeable membrane. Osmotic pressure gradient is the amount of force needed to keep water from flowing across that membrane. Water likes things to be equal on either side of a membrane. It doesn't want one solution to have lots of solute while another has only a little. Therefore, water will rush across a semipermeable membrane from areas of low solute concentration (high water concentration) to areas of high solute concentration (low water concentration). Basically, water wants to dilute that solute.
Is a sulfate solution hypotonic or hypertonic?
Second, it may be considered a hyperto nic solution, meaning it has more solute and less water than another solution. Third, it may be considered a hypotonic solution, meaning it has less solute and more water than another solution.
What is hypotonic solution?
In these arenas, hypotonic refers to a solution ’s having less osmotic pressure, or concentration, than another solution between a semi-permeable membrane. In more simpler terms, hypotonic can mean a solution that has a lower concentration of solutes than other solutions, made of the same solutes.
What does hypotonic mean in biology?
Its many definitions apply to both human biology and biochemistry. When referring to humans or animals, hypotonic signifies a muscle ’s having less tone, or shape, compared to a normal comparison model or when compared to another muscle in the same human body.
What is the difference between a solution and a liquid?
Solution – A liquid with a substance dissolved in it, to the degree that the substance evenly occupies the whole liquid. Osmotic pressure – The difference in concentration of a solution on either side of a semipermeable membrane.
Which solution has the lower concentration of salt?
When two saltwater solutions are placed side-by-side, the solution with the lower concentration of salt is hypotonic to the solution with the higher concentration of salt. 2.
Is tricep muscle hypotonic?
You may then decide to look for exercises that build your triceps up to the same glory as your biceps but, until they reach that state, they will have less tone than their counterparts. Because they have less tone, they are considered hypotonic to your bicep muscle.
Is water a solution or a mixture?
Because sports drinks are essentially a mixture of salt, vitamins, and minerals in water, they are a solution. The mixture is called the solute. Plain water does not have the solute found in sports drinks, however. While science considers water a solution because it is a mixture of H 2 O molecules, it does not contain a mixture of extra salts, ...
Is a raisin hypotonic?
D is correct. The inside of a raisin is hypotonic to exterior water solutions until enough water solution passes through the raisin’s semipermeable membrane so as to make the pressure equal on either side of the membrane.
What is hypotonic solution?
Therefore, a Hypotonic Solution is a solution that contains less solute than blood normally contains. The solute will include various electrolytes, proteins, dissolved blood gases, etc. ( Want a quick refresher on the difference between solute, solvent, and solutions?)
What happens when you administer a Hypotonic Solution?
If you want to understand what happens when you give a patient a hypotonic solution, it all comes down to osmosis and diffusion.
How does hypotonic fluid work?
Let’s do a thought experiment to illustrate how hypotonic fluids work. If we gave a hypotonic IV fluid to a healthy patient without any blood imbalances , then the hypotonic fluid would end up diluting their bloodstream.
Why do you give 0.45% saline?
Typically, if a patient needs a hypotonic solution then they will receive 0.45% Saline. The most common reason to give 0.45% Saline is probably for true dehydration. True dehydration occurs when the body has lost only water, without losing any electrolytes (this is different from fluid volume deficit, where the body loses BOTH water AND electrolytes).
Why would electrolyte imbalances in the hypo direction occur?
hyponatremia, hypokalemia, etc) because there is now more water than “stuff” in the intravascular space.
What happens when the concentration of blood in the blood vessels is reduced?
This will cause water to start moving back into the red blood cells via osmosis.
Do solutions have to be compared to another solution?
Solutions ALWAYS have to be compared to another solution in order to determine their tonicity (hypo-, hyper-, or iso-).
What is hypotonic elevator?
Hypotonic Definition: Imagine you and different humans are anticipating an elevator inside the foyer of a building. When the elevator doorways open, you notice there are 20 humans stuffed into that tiny area.
What is the quantity of solute in an answer?
We name these things solute. The quantity of solute in an answer determines how that answer will react whilst inside the presence of any other answer. An answer may be classified as one in all 3 approaches whilst it’s miles in comparison to any other answer.
Is an answer isotonic or isotonic?
An answer may be classified as one in all 3 approaches whilst it’s miles in comparison to any other answer. First, it could be taken into consideration an isotonic answer, which means it has an identical quantity of solute and water whilst in comparison to any other answer.
