
Valency of First 30 Elements
| Element | Atomic Number | Valency |
| Valency of Hydrogen | 1 | 1 |
| Valency of Helium | 2 | 0 |
| Valency of Lithium | 3 | 1 |
| Valency of Beryllium | 4 | 2 |
What is the difference between the atomic number and valence electrons?
Apr 13, 2020 · Atomic number is equal to total number of protons present in an atom while Valency is number of electrons required in the outermost shell of an atom to become stable. Whereas Valancy is No. of electrons required to complete Octate.
How to find the valence of an atom?
How do you find atomic number from valency? Mathematically we can say that if the outermost shell of an atom contains 4 or less than 4 electrons, then the valency of an element is equal to …
What is valency and how to calculate it?
Atomic number is the total number of electrons. The valence electrons are only those in the outer shell. What is the valency of an atom? Valency is the combining power of an element. …
What is the atomic number of an atom?
31 rows · An atom’s valence is equal to the number of electrons in the outer shell when that number is ...
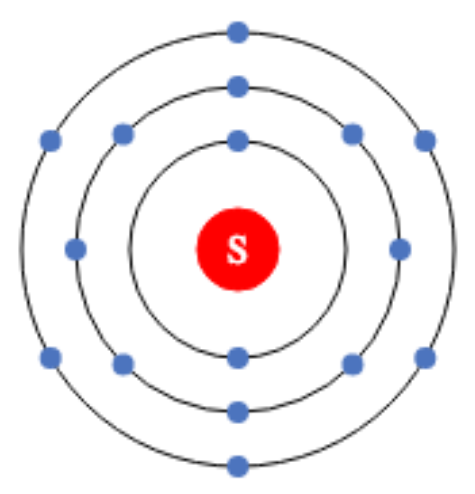
Is atomic number equal to valency?
...
Valency.
| Name of Element | Oxygen |
|---|---|
| Symbol | O |
| Atomic Number | 8 |
| Protons | 8 |
| Neutrons | 8 |
What is the relation between atomic number and valency?
What is difference between valency and atomic mass?
What is the atomic number?
What is called valency?
How do you find the valency and atomic number?
What is difference between atomic number and atomic mass number?
What is difference atomic number and atomic mass?
| Difference Between Atomic Mass and Atomic Number | |
|---|---|
| It is the average weight of an element. | It is the total number of protons in the atom's nucleus. |
| Atomic mass is denoted by A | The letter Z is used to represent an atomic number. |
What is the difference between atomic number and atomic weight?
What is valency class 9th?
What is atomic number example?
What is the atomic number of CA?
What is the valency of all elements in the same group?
For example, all the elements in group 8 have 8 electrons and completely filled orbitals, that is why the valency of all the elements in this group is zero .
How to find the valence of an atom?
How can we calculate Valency? An atom’s valence is equal to the number of electrons in the outer shell when that number is four or fewer. Then the valence in the outer shell is equal to eight minus the number of electrons. Once you are aware of the number of electrons you can calculate the valence easily.
What is the difference between valency and oxidation number?
Difference between Valency and Oxidation Number. Valency is different from the oxidation number, and it has NO SIGN. Thus, the valency of nitrogen is 3, whereas it can have oxidation numbers from -3 to +5. The oxidation number is the hypothetical charge of an atom in a molecule or ion, and it is a measure of its apparent capacity to gain ...
What is the combining capacity of an atom?
The combining capacity of an atom is known as its valency. The number of bonds that an atom can form as part of a compound is expressed by the valency of the element.
How are the number of bonds that an atom can form as part of a compound expressed?
The number of bonds that an atom can form as part of a compound is expressed by the valency of the element. We all know how electrons in an atom are arranged in shells/orbitals. Valence electrons are those electrons which are present in the outermost orbit of the atom.
How many electrons are in the outermost shell of an atom?
From the Bohr-bury scheme, we can say that the outermost shell can contain a maximum of 8 electrons. Only a little chemical activity is observed when the outermost shell is completely filled.
Why are noble gases less reactive?
Noble gases have a completely filled outermost shell and that’s why they are least reactive. Other element’s reactivity depends upon their ability to attain the noble gas configuration. In this section, we shall learn more about the valency of an atom.
What is the atomic number of an element?
Atomic number is usually the number of protons present in an element’s nucleus. It is the average weight of an element. It is the total number of protons in the atom’s nucleus. The letter Z is used to represent an atomic number. Atomic mass cannot be be used to define the type of element.
How are atomic mass and atomic number related?
Atomic mass and an atomic number of elements are said to closely related because whenever the atomic number is high, the atomic mass is also said to be high.
What is the difference between atomic mass and atomic weight?
According to the definitions given, atomic mass which is also called atomic weight is the measured total mass of an element’s atom. Whereas, atomic mass is nothing but the total number of neutrons and protons in the nucleus of an atom.
What is the letter Z in chemistry?
It is the average weight of an element. It is the total number of protons in the atom’s nucleus. The letter Z is used to represent an atomic number. Atomic mass cannot be be used to define the type of element. Atomic number mainly helps in the classification and identification of an element.
Why is atomic mass important?
Atomic number mainly helps in the classification and identification of an element. Atomic mass is also used to classify different isotopes of the same element. Isotopes only share the same atomic number. Atomic mass is mostly measured using atomic mass unit (amu). Atomic number is just a digit that is used to place elements in a periodic table.
Why do we use atomic numbers?
Atomic number mainly helps in the classification and identification of an element. Isotopes only share the same atomic number. Atomic mass is mostly measured using atomic mass unit (amu). Atomic number is just a digit that is used to place elements in a periodic table.
What is the atomic mass unit used for?
Atomic mass is also used to classify different isotopes of the same element. Isotopes only share the same atomic number. Atomic mass is mostly measured using atomic mass unit (amu). Atomic number is just a digit that is used to place elements in a periodic table.

Atomic Number
Representation of Atomic Number
Atomic Numbers' Importance
Atomic Mass
Periodic Table
Valency
- The valency of an atomic orbit is the number of unpaired electrons in the outer shell. The valency of an element is the last unpaired electron in an electronic configuration. The atomic number of sodium, for example, is 11, and the valency is determined by the number of last unpaired electrons, with the unpaired electron numbering one. As a result,...
Things to Remember
Sample Questions