Is FCC or BCC stronger?
BCC metals are infact stronger than FCC metals. HCP metals are the most brittle.
What is a face-centred cubic structure?
Including: FCC. FCC Stands for Face Centred Cubic. It is a type of atomic arrangement and is relatively "tightly" packed (atomic packing factor = 0.74). FCC is formally defined as a cubic lattice with the face positions fully equivalent to each of the eight corners.
What is the difference between face Centred and end Centred unit cell?
Solution : A face-centred unit cell has one constituent particle present at the centre of each face in addition to the particels present at the corners.
A end-centred unit cell has one constituent particle present each at the centre of any two opposite faces in addition to the particels present at the corners.
Why is it called face-centered cubic?
This crystal structure is known as face-centered cubic and has atoms at each corner of the cube and six atoms at each face of the cube.
What is an example of body Centred cubic?
Examples of metals with the bcc structure are alpha iron, tungsten, chromium, and beta titanium.
What is the difference between face Centred cubic and face Centred tetragonal?
Answer: One unit cell of a face-centered cubic has 8 lattice points are corners and 6 lattice points at faces, total 14 lattice points. Answer: One unit cell of face-centered tetragonal has 8 lattice points are corners and 6 lattice points at faces, total 14 lattice points.
How many face-centered cubic unit cell?
six facesA cubic unit cell has six faces. Therefore, in a cubic lattice irrespective of its nature, a cubic unit cell is shared equally by 6 unit cells.
Which unit cell is a face-centered cubic unit cell?
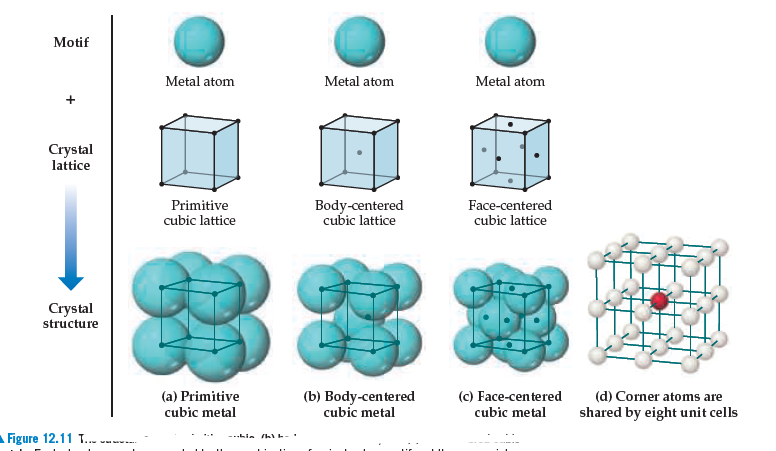
This arrangement is called a face-centered cubic (FCC) solid. A FCC unit cell contains four atoms: one-eighth of an atom at each of the eight corners (8×18=1 atom from the corners) and one-half of an atom on each of the six faces (6×12=3 atoms from the faces).
Is an example of face-centred cubic cell?
An example of a face centred cubic lattice is. Solution : Zn is hcp, Na and CsCl are bcc while Cu is ccp or fcc.
What is another name for face-centered cubic?
Close Packed Structures. Face Centered Cubic (fcc) or Cubic Close Packed (ccp) These are two different names for the same lattice. We can think of this cell as being made by inserting another atom into each face of the simple cubic lattice - hence the "face centered cubic" name.
How do you draw a face-centered cubic structure?
3:1612:16The Body-centered Cubic Structure - YouTubeYouTubeStart of suggested clipEnd of suggested clipFor the body centered cubic structure atoms are touching along the body diagonal. And the bodyMoreFor the body centered cubic structure atoms are touching along the body diagonal. And the body diagonal runs from a front vertex at the bottom say to a back vertex at the top on the opposite.
Body-Centered Cubic Cells
- Some metals crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and an atom in the center, as shown in Figure 2. This is called a body-centered cubic (BCC) solid. Atoms in the corners of a BCC unit cell do not contact each other but contact the atom in the center. A BCC unit cell contains two atoms: one-eighth ...
Face-Centered Cubic Cells
- Many other metals, such as aluminum, copper, and lead, crystallize in an arrangement that has a cubic unit cell with atoms at all of the corners and at the centers of each face, as illustrated in Figure 3. This arrangement is called a face-centered cubic (FCC) solid. A FCC unit cell contains four atoms: one-eighth of an atom at each of the eight corners (8 × 1818 = 1 atom from the corn…
Key Concepts and Summary
- The structures of crystalline metals and simple ionic compounds can be described in terms of packing of spheres. Metal atoms can pack in primitive cubic, body-centered cubic, and face-centered cubic structures. Each packing has its own characteristics with respect to the volume occupied by the atoms and the closeness of the packing.
Glossary
- body-centered cubic (BCC) solid
1. crystalline structure that has a cubic unit cell with lattice points at the corners and in the center of the cell - body-centered cubic unit cell
1. simplest repeating unit of a body-centered cubic crystal; it is a cube containing lattice points at each corner and in the center of the cube