
The key difference between localised and delocalised chemical bonds is that localised chemical bond is a specific bond or a lone electron pair on a specific atom
Atom
An atom is the smallest constituent unit of ordinary matter that has the properties of a chemical element. Every solid, liquid, gas, and plasma is composed of neutral or ionized atoms. Atoms are extremely small; typical sizes are around 100 picometers (1×10⁻¹⁰ m, a ten-milliont…
What is the difference between localised and delocalised chemical bonds?
These regions have a concentrated electron distribution. In other words, the electron density of this region is very high. A localised bond forms when two molecular orbitals of two separate atoms overlap with each other. Sigma bonds may form due to the overlap of two s orbitals, two p orbitals or s-p overlap. What are Delocalised Chemical Bonds?
What is localized chemical bonding?
“What is LOCALIZED chemical bonding?” And, reasonably, this is defined as a two-centre, two-electron bond, the which features a region of HIGH electron density BETWEEN two positively-charged nuclei such that internuclear repulsion is negated, and a net attractive force operates between the positively-charged nuclei, and the electron-rich orbitals.
What is the difference between localized electrons and delocalized electrons?
Localized electrons exhibit normal behavior, a localized lone pair remains close to one atom, and a localized bond pair travels between two atoms. Resonance hybrids necessarily contain some "abnormal" electrons. In a delocalized pi bond, instead of sticking near one atom, it visits two atoms. Beside above, what is a delocalized bond?
How to determine whether lone pairs are localized or delocalized?
On one hand, it looks to be sp3 since it only has single bonds, the steric number is 4 indicating a tetrahedral geometry. To summarize, when you are asked to determine whether the lone pairs localized or delocalized, you need to check which ones can be involved in resonance transformations and which cannot.
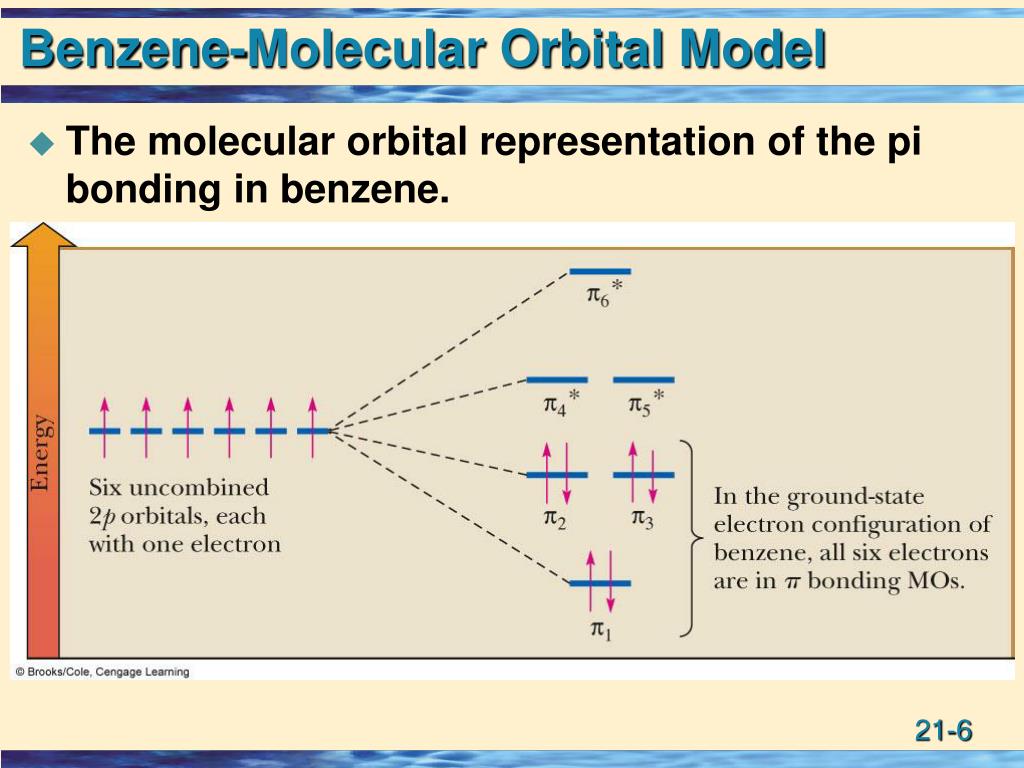
What is the difference between localized and delocalized?
In general chemistry, localized electrons and delocalized electrons are terms that describe chemical structures of chemical compounds. Localized electrons are the bonding electrons in molecules while delocalized electrons are nonbonding electrons that occur as electron clouds above and below the molecule.
How can you tell the difference between localized and delocalized electrons?
If the lone pairs can participate in forming resonance contributors – they are delocalized, if the lone pairs cannot participate in resonance, they are localized.
What is localized and delocalized bond with example?
This type of bond is described as a localised bond. Delocalised bonding electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond. For example, in Benzene molecule, the delocalisation of electrons is indicated by circle.
What is localized bonding?
The localized bonding model (called valence bond theory) assumes that covalent bonds are formed when atomic orbitals overlap and that the strength of a covalent bond is proportional to the amount of overlap.
What bonds have delocalized electrons?
A delocalized electron is an electron in an atom, ion, or molecule not associated with any single atom or a single covalent bond. In a ring structure, delocalized electrons are indicated by drawing a circle rather than single and double bonds.
What is meant by delocalized electrons?
In chemistry, delocalized electrons are electrons in a molecule, ion or solid metal that are not associated with a single atom or a covalent bond.
What is the example of Localised bond?
Sigma bond, pi bond, lone pair. here is an example of H2 . In this molecule , the two H atoms have a sigma bond formed due to end to end overlap. the electronic density lies in between the two H atoms and is restricted there, so this type of bond is a localized chemical bond.
Are pi bonds delocalized?
For example, consider the π bond in ethylene. Before the p orbitals overlap, the p electrons are confined to (or "localized" on) each carbon atom. However, the electrons in the π bond are free to roam over both carbon atoms. That is, the electrons in the π bond are "delocalized".
Lone Pairs and Resonance Stabilization
Localized and Delocalized Lone Pairs
- Now, leaving aside the chemical terminology, in simpler words, one pair of electrons can move around, while the other pair cannot. These electrons belong to only one atom – they are localized. The ones that can move around are delocalized– they can be placed on one atom, but it can also be shared between that and the neighboring atom, i.e. can part...
Hybridization and Delocalization
- Now that we have learned how to classify electrons are localized or delocalized, let’s understand the geometry of the elements participating in delocalization. One of to visualize delocalization is that electrons flow though the orbitals of adjacent atoms. These electrons can be non-bonding (lone pairs) or bonding electrons. More specifically they must be either non-bonding or π bond el…