Answer: The two differences are: 1) the hardening/strengthening effect is not retained at elevated temperatures for precipitation hardening--however, it is retained for dispersion strengthening; and 2) the strength is developed by a heat treatment for precipitation hardening--such is not the case for dispersion strengthening. Advertisement
What is precipitation hardening?
Precipitation or dispersion hardening is one of the important strengthening mechanisms in creep-resistant steels at elevated temperature.
What is the precipitate hardening in creep resistance?
Some particle or precipitate strengthening remains but the majority of the strengthening arises from the dislocation debris left around the particles giving rise to high work hardening. Precipitation or dispersion hardening is one of the important strengthening mechanisms in creep-resistant steels at elevated temperature.
What is the difference between precipitation strengthening and solution strengthening?
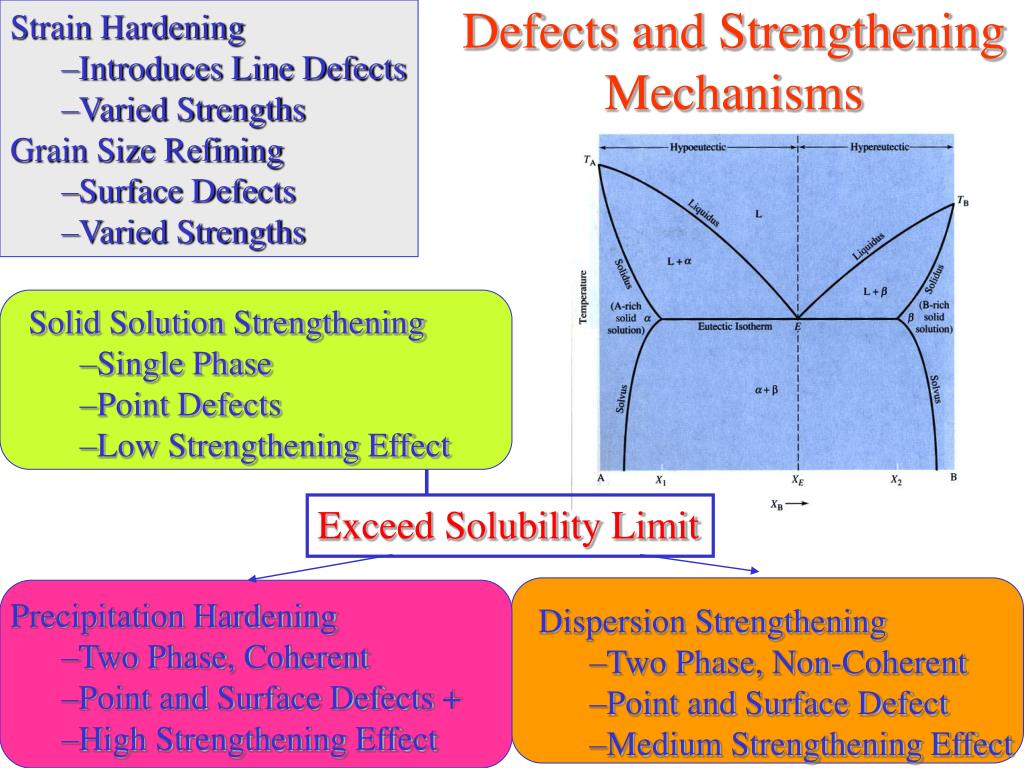
**Solid solution strengthening is the effect of alloying a metal while remaining within the single phase region of the phase diagram. Precipitation strengthening results when the addition is greater than its solubility in the host matrix. Solid solution strengthening is a type of alloying that can be used to improve the strength of a pure metal.
What is the mechanism of hardening and strengthening?
For both dispersion hardening and precipitation hardening, strengthening is due to dislocation motion in the metal being impeded by the presence of small, hard particles. This mechanism operates efficiently only when they are very closely spaced (<1 μm apart).

What does dispersion strengthening do?
Dispersion strengthening refers to the process of increasing the toughness of a metallic object by introducing a second phase through the addition of an alloying element. This is done to increase the strength of metallic objects subject to corrosion.
What is the difference between precipitation hardening and dispersion hardening?
The two differences are: 1) the hardening/strengthening effect is not retained at elevated temperatures for precipitation hardening--however, it is retained for dispersion strengthening; and 2) the strength is developed by a heat treatment for precipitation hardening--such is not the case for dispersion strengthening.
What is the difference between solid solution strengthening and precipitation strengthening?
Differences: **Solid solution strengthening is the effect of alloying a metal while remaining within the single phase region of the phase diagram. Precipitation strengthening results when the addition is greater than its solubility in the host matrix.
What is meant by dispersion hardening?
Dispersion hardening or strengthening is a technique whereby hard or soft external particles are introduced into the aluminium alloy matrix.
What are dispersion strengthened composites?
Dispersion-strengthened composites are materials in which crystalline reinforcement particles, e.g., alumina or zirconia, are dispersed throughout a glass matrix, e.g., a feldspathic glass.
Is precipitation hardening age hardening?
Precipitation hardening, also known as age hardening and particle hardening, is a heat treatment process that is applied to increase yield strength of malleable materials, such as aluminium, magnesium and some select stainless steel grades.
What is precipitation hardening stainless steel used for?
Due to the high strength of precipitation hardening stainless steels, most applications are in aerospace and other high-technology industries. Applications include: Gears. Valves and other engine components.
Can precipitation hardening be reversed?
Manufacturing processes may result in the premature start of the final precipitation age hardening process, which can be reversed through re-solution treating prior to further processing.
What is the difference between solution and precipitation heat treatment?
Solid solution strengthening involves formation of a single-phase solid solution via quenching. Precipitation heat treating involves the addition of impurity particles to increase a material's strength.
What is meant by dispersion?
Dispersion is a statistical term that describes the size of the distribution of values expected for a particular variable and can be measured by several different statistics, such as range, variance, and standard deviation.
What is orowan strengthening?
Orowan strengthening mechanism. Orowan strengthening, caused by the resistance of closely spaced hard particles to the passing of dislocations, is important in aluminium alloys.
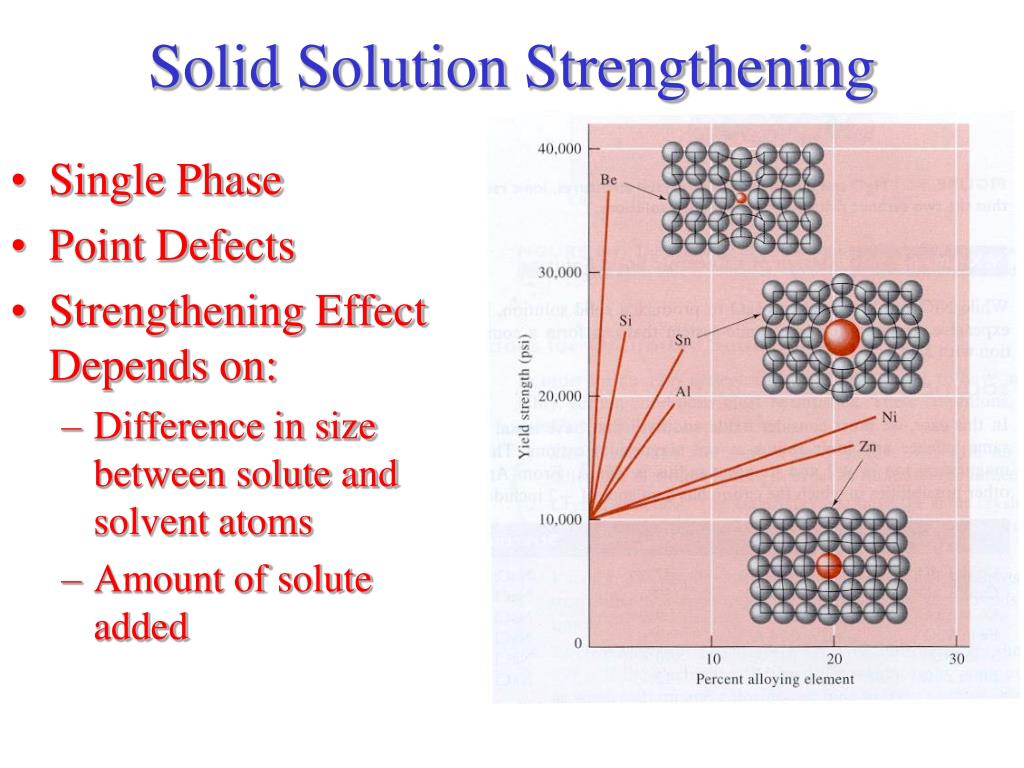
What are two important factors that affect solid solution hardening?
Solid solution strengthening depends on: Concentration of solute atoms. Shear modulus of solute atoms. Size of solute atoms.
What is precipitation hardening of Aluminium?
Precipitation hardening is one heat treatment process by which aluminum alloys can be strengthened in a variety of ways. And this process, also known as artificial aging, is actually performed after a previous round of solution heat treatment and quenching.
What are two important factors that affect solid solution hardening?
Solid solution strengthening depends on: Concentration of solute atoms. Shear modulus of solute atoms. Size of solute atoms.
What is age hardening?
Age hardening is a heat-treatment process used to strengthen metal alloys. Unlike ordinary tempering, alloys must be kept at elevated temperature for hours, or "aged," to allow precipitation to take place.
Do all metals work harden?
Alloys not amenable to heat treatment, including low-carbon steel, are often work-hardened. Some materials cannot be work-hardened at low temperatures, such as indium, however others can be strengthened only via work hardening, such as pure copper and aluminum.
Precipitation Strengthening of a Generalized Binary Alloy
The object of precipitation strengthening is to create in a heat-treated alloy a dense and fine dispersion of precipitated particles in a matrix of deformable metal.
Example problem to calculate weight percent for Aluminum alloy
Calculate the theoretical weight percent of the θ phase that could be formed at 27°C (room temperature) when a sample of Al–4.50 wt% Cu alloy is very slowly cooled from 548°C. Assume the solid solubility of Cu in Al at 27°C is 0.02 wt% and that the θ phase contains 54.0 wt% Cu.
What is dispersion hardening?
Dispersion hardening connected with formation of anomalously oversaturated solid solutions of the TM in aluminum during crystallization of melt drops with the TM aluminide precipitation by the following powder treatment or with immediate formation by crystallization of a disperse intermetallic phase, such as Al3Fe, Al3Zr, etc.
What is yield stress in dispersion hardening?
In dispersion hardening it is assumed that the precipitates do not deform with the matrix and that the yield stress is the stress necessary to expand a loop of dislocation between the precipitates. This will be given by the Orowan stress
What is reinforced material?
Reinforced materials based on metals have long been of technological significance. Dispersion hardened metals and precipitation hardening systems were both developed several decades ago. For both dispersion hardening and precipitation hardening, strengthening is due to dislocation motion in the metal being impeded by the presence of small, hard particles. This mechanism operates efficiently only when they are very closely spaced (<1 μm apart). These materials would not, however, generally be classified as true composites. While there is no universally accepted definition of a composite, it is commonly assumed that it is only when load transfer between matrix and reinforcement is significant that the term can properly be applied. When a composite is subjected to an external load, the matrix is relieved of a substantial proportion of that load by the presence of the reinforcement. On this basis, conventional dispersion and precipitation hardened systems are not composites, since they typically contain only around 1% or less of second phase and at such levels the reinforcing constituent cannot significantly reduce the stress borne by the matrix.
What causes a decrease in Orowan stress?
The coarsening of fine precipitates of M 23 C 6, MX and Fe 2 (W, Mo) Laves phase and the dissolution of fine MX to form massive precipitates of Z phase, which have been observed in 9–12Cr steels during creep, cause an increase in λ in Equation (9.1) and hence a decrease in Orowan stress over long periods of time. 3, 4 The coarsening and dissolution of fine precipitates sometimes takes place preferentially in the vicinity of grain boundaries during creep, which promotes the formation of localized weak zone and promotes localized creep deformation near grain boundaries. 9, 10 This results in premature creep rupture and is time and temperature dependent.
How to heat treat magnesium alloys?
This involves solution treating the magnesium at high temperature to dissolve the intermetallic particles in order to release the alloying elements into solid solution. The material is then thermally aged to maximise the tensile strength by precipitation hardening. A typical heat-treatment cycle involves solution treating at about 440 °C, quenching, and then thermally ageing at 180–200 °C for 16–20 h. These heat-treatment conditions are similar to those used to strengthen age-hardenable aluminium alloys. However, the response of magnesium to precipitation hardening is much less effective than aluminium. Only relatively small improvements to the tensile strength of magnesium alloys are gained by precipitation hardening. This is because the density and mobility of dislocations in magnesium is relatively low owing to the small number of slip systems in the hexagonal crystal structure.
How does magnesium increase strength?
The majority of alloying elements used in magnesium increase the strength by solid-solution hardening and dispersion hardening. The alloying elements react with the magnesium to form fine intermetallic particles that increase the strength by dispersion hardening. The three most common intermetallic particles have the chemical composition: MgX (e.g. MgTl, MgCe, MgSn); MgX2 (e.g. MgCu 2, MgZn 2 ); and Mg 2 X (e.g. Mg 2 Si, Mg 2 Sn). These compounds are effective at increasing the strength by dispersion hardening, but they reduce the fracture toughness and ductility of magnesium. For example, the Mg–Al–Mn and Mg–Al–Zn alloys used in aircraft form particles (Mg 17 Al 12) at the grain boundaries which lower the toughness and ductility.
How do dislocations affect metals?
The strength properties increase whilst the ductility decreases with increasing concentration of dislocations. The dislocation density in high-strength metals is typically in the range of 10 12 to 10 14 per cubic centimetre.
When does precipitation strengthening occur?
Precipitation strengthening occurs when the precipitates are coherent with the matrix (crystallographic relationship). The loss of coherency leads to a loss of strengthening.
How to achieve strengthening effect from precipitates?
To achieve the strengthening effect from the precipitates, the alloy is heated above the solution temperature of the second phase, while remaining in the solid state. After a quench, the alloy is aged below the solution temperature.
How do microalloying elements act in stenghtening the materials?
The microalloying elements can act in stenghtening the materials into two ways: - as solute by dragg effect : when interacting with dislocations hindering therefore their motion " solid solution strenghtening ". - as precipitates by pinning effect : "precipitation strenghtening".
What happens when a solute atom is larger than the interstice?
If larger than the interstice (space in between atoms), they create tensile forces at that region, if smaller they induce compressive forces. Substitutional solute atoms replace the solvent atoms in the crystal lattice of the metal.
What is the strengthening effect of solute atoms in a crystal?
The strengthening effect of solute atoms in a crystal depends on the size difference between the solute and the solvent atoms and the concentration of the solute. The more is the difference in the atomic sizes, the more is the strengthening effect.
Which technique is used to harden metals?
The introduction of solute atoms into solid solution in the solvent-atom lattice invariably produces an alloy which is stronger than the pure metal.This technique to strengthen and harden metals is alloying with impurity atoms that go into either substitutional or interstitial solid solution.
What enhances the strain field around them and similar strengthening mechanism is operative?
Interstitial or substitutional elements enhances the strain field around them and similar strengthening mechanism is operative. But here looping/bowing will be applicable.
What is precipitation hardening?
Precipitation hardening, also called age hardening or particle hardening, is a heat treatment technique used to increase the yield strength of malleable materials, including most structural alloys of aluminium, magnesium, nickel, titanium, and some steels and stainless steels. In superalloys, it is known to cause yield strength anomaly providing ...
How does coherency hardening occur?
Coherency hardening occurs when the interface between the particles and the matrix is coherent, which depends on parameters like particle size and the way that particles are introduced . Small particles precipitated from supersaturated solid solution usually have coherent interfaces with the matrix. Coherency hardening originates from the atomic volume difference between precipitate and the matrix, which results in a coherency strain. The associated stress field interacts with dislocations leading to an increase in yield strength, similar to the size effect in solid solution strengthening.
How does precipitation affect dislocation?
Dislocations are repulsed by regions of higher stiffness. Conversely, if the precipitate causes the material to be locally more compliant, then the dislocation will be attracted to that region. In addition, there are three types of interphase boundaries (IPBs).
How does order strengthening occur?
Order strengthening occurs when the precipitate is an ordered structure such that bond energy before and after shearing is different. For example, in an ordered cubic crystal with composition AB, the bond energy of A-A and B-B after shearing is higher than that of the A-B bond before. The associated energy increase per unit area is anti-phase boundary energy and accumulates gradually as the dislocation passes through the particle. However, a second dislocation could remove the anti-phase domain left by the first dislocation when traverses the particle. The attraction of the particle and the repulsion of the first dislocation maintains a balanced distance between two dislocations, which makes order strengthening more complicated.
What is chemical strengthening?
Chemical strengthening is associated with the surface energy of the newly introduced precipitate-matrix interface when the particle is sheared by dislocations. LIke modulus hardening, the analysis of interfacial area can be complicated by dislocation line distortion.
What causes lattice distortion?
The presence of second phase particles often causes lattice distortions. These lattice distortions result when the precipitate particles differ in size and crystallographic structure from the host atoms. Smaller precipitate particles in a host lattice leads to a tensile stress, whereas larger precipitate particles leads to a compressive stress. Dislocation defects also create a stress field. Above the dislocation there is a compressive stress and below there is a tensile stress. Consequently, there is a negative interaction energy between a dislocation and a precipitate that each respectively cause a compressive and a tensile stress or vice versa. In other words, the dislocation will be attracted to the precipitate. In addition, there is a positive interaction energy between a dislocation and a precipitate that have the same type of stress field. This means that the dislocation will be repulsed by the precipitate.
Why does nucleation occur at a high temperature?
Nucleation occurs at a relatively high temperature (often just below the solubility limit) so that the kinetic barrier of surface energy can be more easily overcome and the maximum number of precipitate particles can form. These particles are then allowed to grow at lower temperature in a process called ageing. This is carried out under conditions of low solubility so that thermodynamics drive a greater total volume of precipitate formation.