Main Differences Between Pure Substance and Mixture
- A pure substance is a substance made up of one component whereas a mixture is a substance that is made up of the combination of two or more components.
- The chemical and the physical properties of the pure substance remain the same throughout but they change in a mixture.
What is the difference between pure substances and mixtures?
• Pure substance cannot be separated into two or more substances by any mechanical or physical method. In contrast, mixtures contain two or more substances, so they can be separated. • Pure substances are homogenous; therefore, the properties are uniform throughout the sample. Mixtures show the properties of the pure substances in it.
What are some examples of pure substances and mixtures?
they can be separated into other substances by some physical methods. hydrogen, pure water, gas, and gold are common examples of pure substances. sugar, oil, sand, and water are common examples of mixtures. due to the single type of substance, the composition remains the same throughout.
What are pure substances and mixtures?
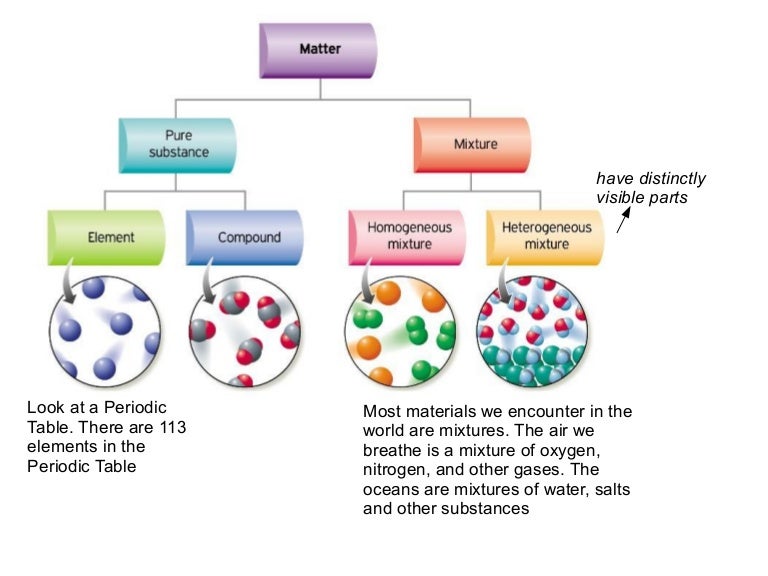
Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Pure substances are further divided into elements and compounds. The combination of two or more pure substances is called a mixture. Mixtures can be classified into two types viz. heterogeneous and homogeneous mixtures.
Is a mixture a pure substance?
The Difference Between a Mixture and a Pure Substance. A mixture is a compound composed of two or more types of pure substances. A pure substance is the same regardless of its composition. A mixture is not pure; the components are chemically bound together. A mixture can only be separated by chemical or physical processes.
What is the combination of two or more pure substances called?
The combination of two or more pure substances is called a mixture. Mixtures can be classified into two types viz. heterogeneous and homogeneous mixtures. A few differences between pure substances and mixtures have been tabulated below.
What is the difference between pure substances and mixtures?
Solid, liquid and gas are the three states of matter. However, matter can also be classified into pure substances and mixtures. Substances which have a specific composition and cannot be separated into any constituents are called pure substances. Pure substances are further divided into elements ...
What is a mixture of substances?
A substance is a collection of like particles - all one element or compound. A mixture is multiple substances mingled together.
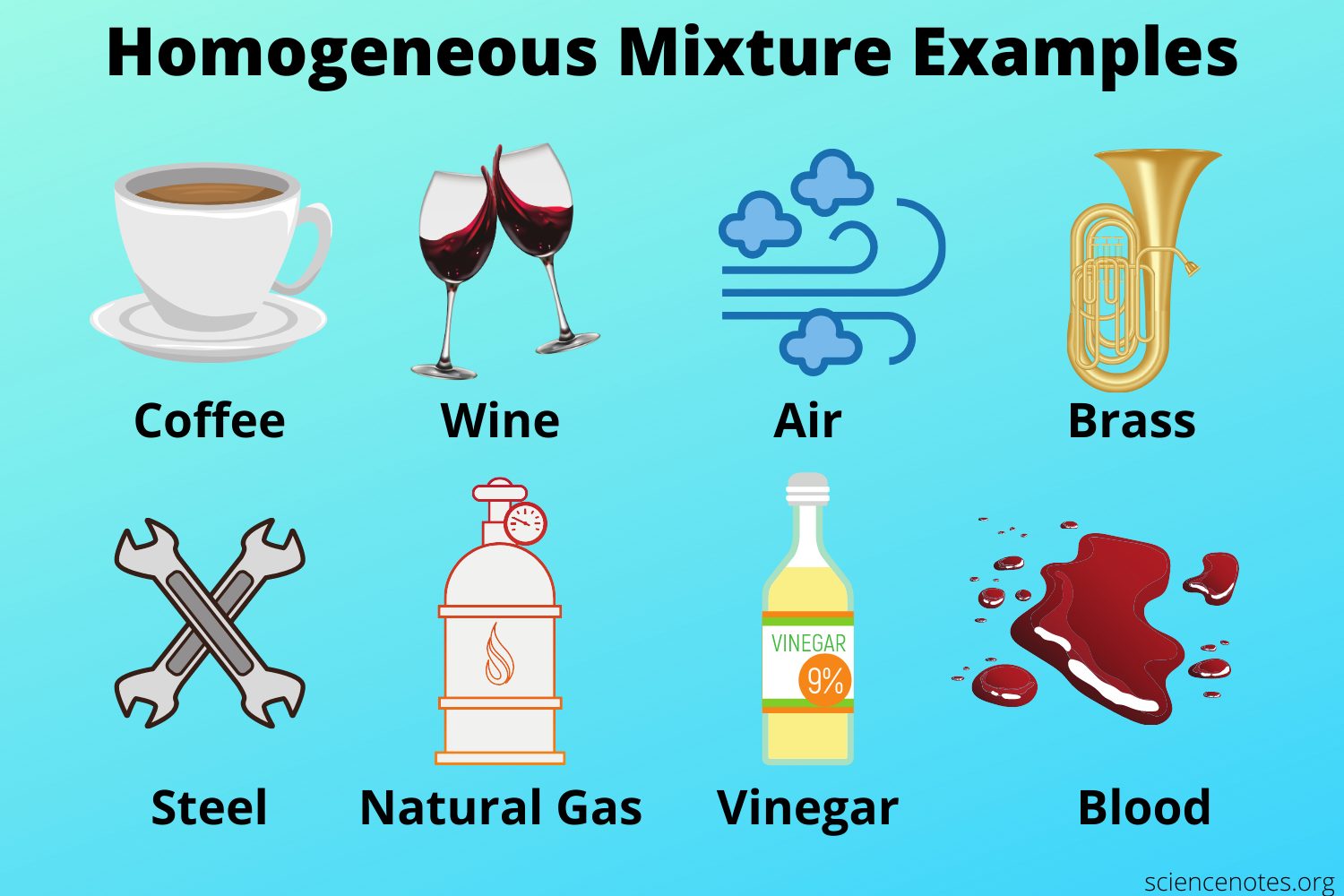
Is saltwater a homogeneous mixture?
Alloys of metals and mixtures of gases such as air are other examples of homogeneous mixtures. Soil and sand in water are examples of heterogeneous mixtures.
What are the two elements that make up life?
There aren’t many things in life that are not made up of at least two elements. For instance, iodized water, arguably one of the most important sources of life, is a union of two elements – Hydrogen and Oxygen.
Can a substance be divided into two groups?
Since they are formed by ionic procedures, they can only be divided by ionic operations equally. Generally, substances can be subdivided into two groups; elements and compounds. Example of substance and mixture include alloys, iodized water, etc.
What are the properties of a mixture?
Based on their composition, mixtures get divided into the homogeneous and heterogeneous mixture. Physical properties. Physical properties of pure substances are definite and constant. Mixtures have varying physical properties. Chemical properties. Chemical properties of pure substances are constant and definite too.
What is it called when two or more substances come together?
When two or more pure substances come together, we call it a mixture . Mixtures can get divided into two kinds, namely, homogeneous and heterogeneous mixtures. In this article, you will learn about the difference between pure substance and mixture and fundamental concepts of the same.
What is a heterogeneous mixture?
Heterogeneous Mixtures – Those mixtures which do not have a uniform composition entirely are heterogeneous mixtures. A mixture of oil and water, soil and sand are heterogeneous mixtures as they don’t have a uniform composition.
What is a compound?
A compound refers to a matter consisting of two or more different elements joined together chemically. You must know that a molecule forms when two or more atoms or elements get bonded chemically. Those elements might belong to the same type or different ones. For instance, O2 is a diatomic molecule because it has two oxygen molecules which come together chemically. But, CO2 is a compound because it has two different atoms. It has carbon and oxygen which got bonded chemically.
What are the two categories of substances?
So as you know, all the substances can get divided into two categories – pure substance and mixture. Here, we will study both categories.
What are the three fundamental states of matter?
Solid, liquid, and gas are the three fundamental states of matter. However, you can classify the matter in pure substances and mixtures as well. Substances that have a particular composition get called as pure substances. They cannot get separated into other forms of matter.
What is a mixture?
Mixture. Definition. The substances composed of only one particular matter are pure. When two or more forms of matter come together to form a substance, it becomes a mixture. Categories. Based on their composition, pure substances get classified into elements and compounds.
What is the product of a mechanical blending or mixing of chemical substances like elements and compounds?
Mixtures are the one product of a mechanical blending or mixing of chemical substances like elements and compounds, without chemical bonding or other chemical change, so that each ingredient substance retains its own chemical properties and makeup.
What is a mixture in chemistry?
In chemistry, a mixture is a material system made up of two or more different substances which are mixed but are not combined chemically. A mixture refers to the physical combination of two or more substances on which the identities are retained and are mixed in the form of solutions, suspensions, and colloids.
What are the two categories of matter?
Matter can be broken down into two categories: pure substances and mixtures. Pure substances are further broken down into elements and compounds. Mixtures are physically combined structures that can be separated into their original components.
Why do some substances form colloids?
Whereas some substances form colloids because molecules cluster to form colloidal particles, in polymers and, in particular, proteins, the molecules are large enough that they form colloidal particles on their own .
Why are substances called pure?
Chemical substances are often called 'pure' to set them apart from mixtures. A common example of a
What is a chemical substance?
A chemical substance is a form of matter that has constant chemical composition and characteristic properties. It cannot be separated into components by physical separation methods, i.e., without breaking chemical bonds. Chemical substances can be chemical elements, chemical compounds, ions or alloys.
What is mixture in science?
To quote my fifth grade science lesson: a mixture is a combination of things that is reservable, and the things combined can be taken out again, for example, a cereal or a salad is a mixture of things, and the individual parts can be identified and taken out. I think the other word you are looking for is solution, which is a combination of things that is irreversible (i.e., salt and water)
Why can hexane be separated from water?
For example, hexane can be separated from a mixture of hexane and water, because hexane boils and evaporates before water does. The amount of substances in a mixture can vary, and these amounts do not have a fixed ratio. Therefore, even two mixtures containing similar types of substances can be different, due to the difference in their mixing ...
What are the two types of mixtures?
Solutions, alloys, colloids, suspensions are the types of mixtures. Mixtures can be mainly divided into two as homogenous mixtures and heterogeneous mixtures. A homogenous mixture is uniform; therefore, the individual components cannot be separately identified.
What is the difference between pure substance and mixture?
Single elements are hardly stable under natural conditions. They form various combinations among them or with other elements in order to exist. Not only elements, molecules and compounds also tend to mix with a large number of other species in nature.
What is the smallest element?
For example, the smallest element is the hydrogen. Silver, gold, and platinum are some of the commonly known precious elements. Elements can be subjected to chemical changes to form various compounds; however, elements cannot be further broken down by simple chemical methods. Compounds are the other type of pure substances.
Can mixtures be heterogeneous?
Mixtures show the properties of the pure substances in it. Mixtures can be heterogeneous and having a non-uniformity throughout the sample.
Do mixtures have physical properties?
They only have physical interactions. Since they do not have any chemical interactions, in a mixture, the chemical properties of the individual substances are retained without change. But the physical properties like melting point, boiling point can be different in a mixture compared to its individual substances.
Is a substance homogeneous?
Pure substance cannot be separated into two or more substances by any mechanical or physical method. Therefore, pure substance is homogenous. It has a uniform composition throughout the sample. Further, the properties of it are also uniform throughout the sample. Elements are pure substances.
