
What is the difference between electronegativity and electron affinity?
What is the Difference Between Electronegativity and Electron Affinity
- Electronegativity is a numerical value associated with an atoms ability to form a covalent bond
- Electron affinity is the amount of energy that is released when an electron attaches to the atom
- Electron affinity is a fixed and measurable value
What is the value of electron affinity?
The values of electron affinity are given in kJ/mol. Values in parentheses ( ) are predicted values. Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed.
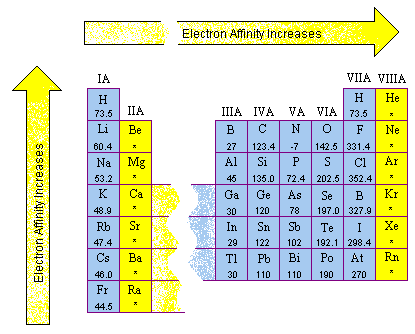
What is the trend of electron affinity on the periodic table?
Periodic Trends
- Across Periods. As we travel from left to right on the periodic table, electron affinities become more positive- meaning that the electron attachment process is more exothermic.
- Down Groups. As we travel down groups, electron affinities become more negative, meaning the process is more endothermic.
- Summary of Trend. ...
What is the first ionization energy of nitrogen?
The ionization energy of molecular nitrogen is 1503 kJ mol-1, and that of atomic nitrogen is 1402 kJ mol-1. Once again, the energy of the electrons in molecular nitrogen is lower than that of the electrons in the separated atoms, so the molecule is bound. What element in Period 2 has the highest first ionisation energy?
Why is nitrogen electron affinity zero?
Electron affinity of Nitrogen is zero due to its half-filled configuration of 2p-orbital, which gives stability to it. Half filled configuration in Nitrogen has gives stability to it, thus its electron affinity is Zero.
Why is the electron affinity of nitrogen positive?
Because the effective nuclear charge overpowers this repulsion, and energy is being released when an electron is being added to oxygen, hence the electron affinity will be positive.
Does nitrogen have high electron affinity?
Nitrogen has lower electron affinity than its preceeding element carbon because. Electron affinity decreases along a period.
How do you calculate electron affinity?
The amount of energy released when a neutral atom in its gaseous state accepts an electron and gets converted into a negatively charged ion is known as electron affinity. X ( g ) + e − → X − ( g ) + E . A . Therefore, the electron affinity of chlorine is – 349 KJ/mol.
Which has highest electron affinity nitrogen or oxygen?
Also, oxygen's electron affinity is greater than nitrogen because oxygen is more electronegative than nitrogen and hence it has more tendency to take electrons.
Which has highest electron affinity?
Hence, among given options chlorine has highest electron affinity.
Which has highest electron affinity and why?
Although Fluorine has the highest electronegativity, Chlorine has the highest electron affinity and this is because the considerable repulsion in the tightly packed 2p subshell of Fluorine (whereas chlorine is an atom with a larger atomic size).
What is 1st and 2nd electron affinity?
There are two types of electron affinity, first and second. The first involves the addition of an electron to a neutral atom. Because this exothermic process releases energy, first electron affinities are negative values. The second pertains to the addition of an electron to a negative ion.
What is the electron affinity of oxygen?
141 kJ/molElectron Affinity of Oxygen is 141 kJ/mol. Electronegativity of Oxygen is 3.44. First Ionization Energy of Oxygen is 13.6181 eV.
What means electron affinity?
electron affinity, in chemistry, the amount of energy liberated when an electron is added to a neutral atom to form a negatively charged ion. The electron affinities of atoms are difficult to measure, hence values are available for only a few chemical elements, chiefly the halogens.
Why does nitrogen have a positive charge?
The nitrogen has five valence electrons. Here, only four are involved in the covalent binding of the -O, =O, and -OH. Only one of the two electrons involved in the covalent bonds belongs to the nitrogen. As there are only four covalente bonds, nitrogen is lacking one electron, therefore it is positively charged.
Why is the electron affinity of N positive while C and O are negative?
The atomic number of carbon is six, and the atomic number of oxygen is 8. Both carbon and oxygen do not have half-filled p orbital. The number of p electrons in carbon and oxygen is two and four, respectively. Therefore, nitrogen has a lower electron affinity than carbon and oxygen.
Why is electron affinity positive or negative?
Unlike ionization energies, which are always positive for a neutral atom because energy is required to remove an electron, electron affinities can be negative (energy is released when an electron is added), positive (energy must be added to the system to produce an anion), or zero (the process is energetically neutral) ...
What does a positive value for electron affinity indicate?
A positive electron affinity means that energy is required for the electron to be added to the atom. A negative electron affinity means that energy is released when the electron is added to the atom. You should know that a greater electron affinity is a more negative value.
How to use electron affinity?
To use electron affinities properly, it is essential to keep track of sign. When an electron is added to a neutral atom, energy is released. This affinity is known as the first electron affinity and these energies are negative. By convention, the negative sign shows a release of energy. However, more energy is required to add an electron to a negative ion which overwhelms any the release of energy from the electron attachment process. This affinity is known as the second electron affinity and these energies are positive.
What is the symbol for electronegativity?
Electronegativity, symbol χ, is a chemical property that describes the tendency of an atom to attract electrons towards this atom. For this purposes, a dimensionless quantity the Pauling scale, symbol χ, is the most commonly used.
What is the meaning of ionization energies?
Note that, ionization energies measure the tendency of a neutral atom to resist the loss of electrons. Electron affinities are more difficult to measure than ionization energies.
How much ionization energy is needed to remove an electron?
Some of these electrons are more tightly bound in the atom than others. For example, only 7.38 eV is required to remove the outermost electron from a lead atom, while 88,000 eV is required to remove the innermost electron. Helps to understand reactivity of elements (especially metals, which lose electrons).
What is the ionization energy of a species?
The n th ionization energy refers to the amount of energy required to remove an electron from the species with a charge of ( n -1) .
How does electronegativity affect an atom?
In general, an atom’s electronegativity is affected by both its atomic number and the distance at which its valence electrons reside from the charged nucleus. The higher the associated electronegativity number, the more an element or compound attracts electrons towards it.
Which atom is the least electronegative?
The most electronegative atom, fluorine, is assigned a value of 4.0, and values range down to cesium and francium which are the least electronegative at 0.7.
Which has more electron affinity, nitrogen or oxygen?
And there are two factors which influences the electron affinity that- as a, all rounder king “effective nuclear charge” which focuses on to attract the electrons adding up to that atom…. In case of Nitrogen (7) and Oxygen (8) ……the oxygen atom have more attracting power of electrons towards its nucleus than nitrogen, so, it will be more electron affinity than nitrogen. And the second factor is electronic state as oxygen have two p orbital unpaired that can accept electron while nitrogen have three p orbital available with unpaired electron to accept electron, so, nitrogen will feel more repulsion while oxygen will ….SO, we can say that from these facts that oxygen will show more electron affinity than that of nitrogen.
How many electrons does nitrogen have?
Nitrogen has five electrons in its valence shell and when electron approaches to Nitrogen then electron loses half of its energy to exist in valence shell and then half of its remaining energy uses to dominate the repulsion of valence electrons . So net energy loss is zero . That is the reason Nitrogen has approximately zero Electron Effinity . And it is also accounted in inert gases.
Why is oxygen smaller than sulfur?
Oxygen being smaller in size than sulfur, has its valence shell electrons placed more closely than sulfur. Due to this electrons repel each other greatly. As an external electron is introduced, energy is released (which is called electron affinity) but at the same time, some of the energy is consumed due to the instability caused by electron-electron repulsion. S0 the overall electron affinity of the oxygen comes out to be lesser than that of sulfur. Same explanation applies to the case of fluorine and chlorine.
What happens when you force an electron on a valence shell?
When you force an electron on this, two things happen, 1. you break the special symmetric case and this requires you put in energy. 2. the electron you just added is attracted to the nucleus (like all the rest) but not by enough to offset the energy you put in to break the symmetry.
Which is smaller, oxygen or sulfur?
Oxygen being smaller in size than sulfur, has its valence shell electrons placed more closely than sulfur. Due to this electrons repel e
Is nitrogen a full or empty shell?
In the case of nitrogen, it has a half-full 2p subshell. While this is not as stable as a full or empty valence shell, it is still more stable than it would be if it gained or lost one or two electrons.
Is noble gas an elemental or atomic state?
except noble gases all elements are found in chemically combined state ( elemental state and with other as well) noble gas exist in mono atomic state the diffrence between noble gases and other elements is noble gases has maximum stablity and minimum energy .
What is electron affinity?
Electron affinity is the amount of energy change (ΔE) that occurs when an electron is added in the outermost shell of an isolated gaseous atom. In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. ...
What is the energy change of an electron?
In other words, when the electron is added to a neutral atom, the energy is either released or absorbed. And this amount of energy change (ΔE) is called electron affinity. This energy change (ΔE) can be positive, negative or zero. And the sign of Electron Affinity (E EA) is opposite to the sign of energy change (ΔE).
Is the sign of electron affinity positive or negative?
Hence the sign of Electron Affinity (EEA) will be positive. For endothermic reaction (i.e when energy is absorbed), the change in energy (ΔE) is positive. Hence the sign of Electron Affinity (EEA) will be negative. For neutral process (i.e when energy is neither absorbed nor released), the value of Electron Affinity (E EA) will be zero.
How many electrons does nitrogen have?
Nitrogen's electron configuration is 1s22s22p3, so all three p orbitals have one electron each. Since electron affinity is associated with the "love" for acquiring another electron, and the new electron would be added in one of the singly-occupied p orbitals, we have a problem.
How many electrons are in a singly occupied orbital?
Each singly-occupied p orbital already has one electron, so that electron, clearly identical to the electron being added in every way--- INCLUDING CHARGE ---will repel the incoming electron, so more energy than usual is needed to add it.
Which has more electron affinity, nitrogen or oxygen?
And there are two factors which influences the electron affinity that- as a, all rounder king “effective nuclear charge” which focuses on to attract the electrons adding up to that atom…. In case of Nitrogen (7) and Oxygen (8) ……the oxygen atom have more attracting power of electrons towards its nucleus than nitrogen, so, it will be more electron affinity than nitrogen. And the second factor is electronic state as oxygen have two p orbital unpaired that can accept electron while nitrogen have three p orbital available with unpaired electron to accept electron, so, nitrogen will feel more repulsion while oxygen will ….SO, we can say that from these facts that oxygen will show more electron affinity than that of nitrogen.
How many electrons does nitrogen have?
Nitrogen has five electrons in its valence shell and when electron approaches to Nitrogen then electron loses half of its energy to exist in valence shell and then half of its remaining energy uses to dominate the repulsion of valence electrons . So net energy loss is zero . That is the reason Nitrogen has approximately zero Electron Effinity . And it is also accounted in inert gases.
Which atom has an open path?
On the other hand, the halogen has a open-path (valance) where the outer electron of a 2nd atom can find and bonding positions where attracting both nucleus in 3D, so bonding. That dual attraction
Is nitrogen a full or empty shell?
In the case of nitrogen, it has a half-full 2p subshell. While this is not as stable as a full or empty valence shell, it is still more stable than it would be if it gained or lost one or two electrons.
Is every orbital in a noble gas fully occupied?
In a noble gas, every orbital from the highest occupied orbital down (up/down refer to energy here) is fully occupied, and every higher energy orbital is completely unoccupied. Gaining an electron changes that so that the highest occupied orbital is only half filled. There are atoms whose highest occupied orbital is a half-filled s orbital: they’re Group 1, called the alkali metals, and very easily ionizable to get to the electron configuration that the noble gas atom started with.
