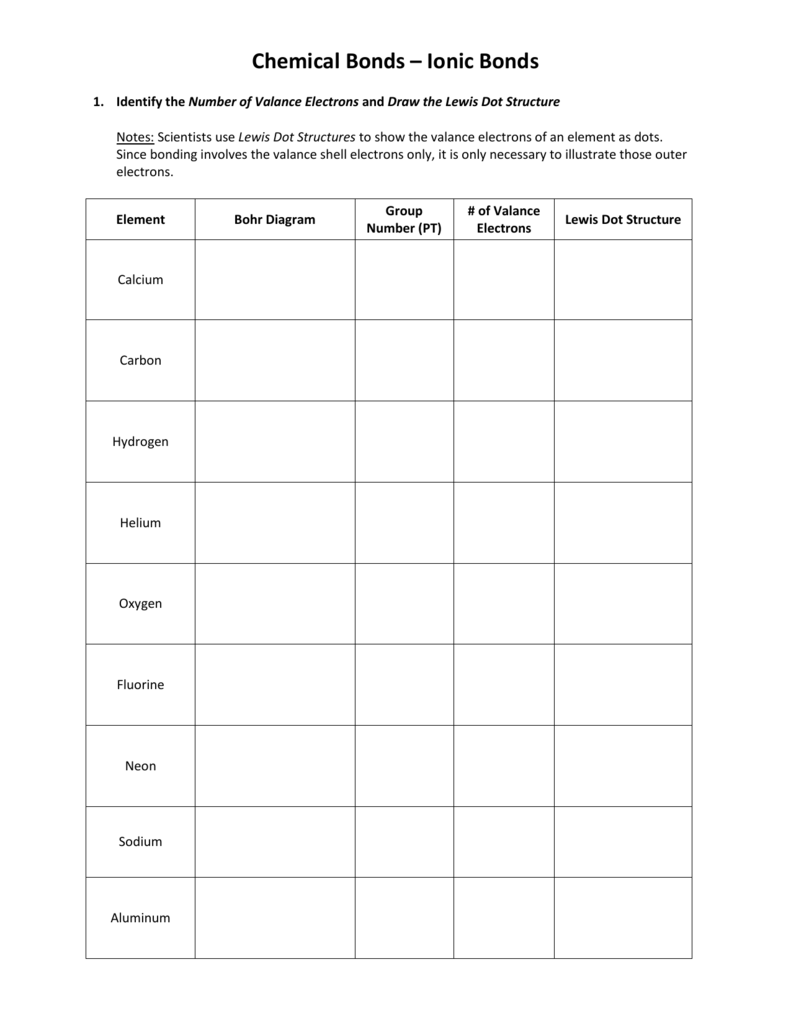
How do you find electron dot diagram?
0:314:18Electron Dot Diagram - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd starting from the top or you can start from any point from the cross. Start to draw in oneMoreAnd starting from the top or you can start from any point from the cross. Start to draw in one electron at a time at each end of the cross.
How many dots are on the Lewis dot structure for neon?
Fluorine and neon have seven and eight dots, respectively: With the next element, sodium, the process starts over with a single electron because sodium has a single electron in its highest-numbered shell, the n = 3 shell.
What is the electron dot diagram for lithium?
A beryllium atom, with two valence electrons, would have the electron dot diagram below. Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired....Electron Dot Diagrams.lithium1s22s11 valence electronneon1s22s22p68 valence electrons2 more rows•Jul 6, 2019
What is the charge for neon?
0Table of Common Element ChargesNumberElementCharge9fluorine1-10neon011sodium1+12magnesium2+88 more rows•Jul 18, 2022
How do Lewis dot diagrams work?
A Lewis diagram shows how the valence electrons are distributed around the atoms in a molecule. Shared pairs of electrons are drawn as lines between atoms, while lone pairs of electrons are drawn as dots next to atoms.
What is the electron dot diagram for Helium?
Lewis Dot Structure of Helium Helium is a noble gas with a complete valence shell. It is denoted by the symbol He. Helium, unlike the other noble gases in Group 8, has only two valence electrons and the first orbit only has those two electrons. The electrons are represented as two lone pair dots in the Lewis symbol.
What is the dot diagram for carbon?
0:241:16Lewis Dot Structure for Carbon (C) - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd then we go three four so one two three four carbon is in group four right here so it has fourMoreAnd then we go three four so one two three four carbon is in group four right here so it has four valence electrons. And you're done that's the lewis dot structure for carbon.
What is the electron dot diagram for hydrogen?
0:091:21H2 Lewis Structure - How to Draw the Dot Structure for H2 - YouTubeYouTubeStart of suggested clipEnd of suggested clipAnd because they're sharing that hydrogen also has two. So that's the dot structure for hydrogen gasMoreAnd because they're sharing that hydrogen also has two. So that's the dot structure for hydrogen gas we could also write it as a structural formula.
How many valence electrons does neon have?
eight valence electronsExplanation: Neon, Z=10 , has eight valence electrons. This closed-shell configuration makes neon supremely difficult to oxidize, and difficult to reduce. The inertness, the lack of reactivity, of this Noble Gas, is a function of its electronic configuration.
How many Lewis dots does oxygen have?
Each O is surrounded by four dots and two sticks or lines, representing another 4 electrons in the O2 double bond. So each O is surrounded by 8 total valence electrons, giving it an octet and making it stable. The two letter O's in the O2 Lewis structure represent the nuclei (centers) of the oxygen atoms.
What is the Lewis dot structure for Argon?
0:001:21Lewis Dot Structure for Argon Atom (Ar) - YouTubeYouTubeStart of suggested clipEnd of suggested clipArgon is in group 18 or 8a that means it has 8 valence electrons. We'll put those 8 valenceMoreArgon is in group 18 or 8a that means it has 8 valence electrons. We'll put those 8 valence electrons around the element symbol ar for argon.
How many dots should occur in the Lewis symbol of a krypton atom?
0:471:21Lewis Dot Structure for Krypton Atom (Kr) - YouTubeYouTubeStart of suggested clipEnd of suggested clipWe put those eight valence electrons around KR the element symbol for Krypton. This is dr. B withMoreWe put those eight valence electrons around KR the element symbol for Krypton. This is dr. B with the Lewis dot structure for Krypton.
What are the valence electrons?
Recall that the valence electrons of an atom are the electrons located in the highest occupied principal energy level. Valence electrons are primarily responsible for the chemical properties of elements. The number of valence electrons can be easily determined from the electron configuration. Several examples from the second period elements are ...
How many valence electrons are in a transition element?
Electron dot diagrams would be the same for each element in the representative element groups. Most transition elements have two valence electrons, though some that have unusual electron configurations have only one.
How many valence electrons are in a s block?
In each case, valence electrons are those in the second principal energy level. As one proceeds left to right across a period, the number of valence electrons increases by one. In the s block, Group 1 elements have one valence electron, while Group 2 elements have two valence electrons.
What is the purpose of diagrams?
Diagrams contain a lot of useful information in a compact format. Diagrams of electrons provide information on the location of specific electrons. We can mark these electrons and indicate what happens to them when an element reacts.
How many valence electrons does a beryllium atom have?
A beryllium atom, with two valence electrons, has the electron dot diagram below: Since electrons repel each other, the dots for a given atom are distributed evenly around the symbol before they are paired. The table below shows the electron dot diagrams for the entire second period.
Answer
The dot structure for Neon shows a full inner shell with 2 electrons and a full outer shell with 8 electrons. So it is unlikely to gain or lose electron (s) in a reaction with other elements. Neon is likely to be chemically inert based on this structure.
Answer
the dot structure shows a full complete outer e- shell with 8 e- so Neon doesnt want more or less e-. its gonna be an inert gas.
