
What experiment proved that the negative charge and Ray are inseparable?
He found that by applying a magnetic field across the tube, there was no activity recorded by the electrometers and so the charge had been bent away by the magnet. This proved that the negative charge and the ray were inseparable and intertwined.
Are electrons negatively charged or positively charged?
The entire concept that electrons are negatively charged is purely a matter of convention. If you take a proton to be positively charged then by nature of charges any charge that repels a proton must be taken positive and any charge that attracts it must be taken negative.
What was Thomson’s first cathode ray experiment?
Thomson’s First Cathode Ray Experiment. Thomson had an inkling that the ‘rays’ emitted from the electron gun were inseparable from the latent charge, and decided to try and prove this by using a magnetic field. His first experiment was to build a cathode ray tube with a metal cylinder on the end.
How did JJ Thomson determine the charge of an electron?
An ebonite rod rubbed with silk or fur acquires a positive charge and the silk acquires a negative charge. As to electrons, they were detected by JJ Thomson. An experiment called "oil drop experiment" determined charge on electron. As the electron is repelled by the nucleus of an atom(which is positively charged due to protons).
How did we find out electrons are negatively charged?
Electrons have a Negative Charge Thomson's cathode ray experiment was able to determine the charge-to-mass ratio of electrons. About ten years later, American physicist Robert A. Millikan performed what is now known as the Millikan oil-drop experiment. This experiment allowed the charge of an electron to be determined.
What was JJ Thomson experiment called?
experiment Cathode Ray Tube (CRT)The experiment Cathode Ray Tube (CRT) conducted by J. J. Thomson, is one of the most well-known physical experiments that led to electron discovery. In addition, the experiment could describe characteristic properties, in essence, its affinity to positive charge, and its charge to mass ratio.
What was JJ Thomson's cathode ray experiment?
Thomson's cathode ray tube experiment discovered the subatomic particle the electron. Prior to the experiment, it was not known that atoms were composed of further particles. Cathode rays were determined to be composed of negatively charged particles that were smaller than the smallest atom.
Who proved electrons are negative?
J.J. Thomson'sJ.J. Thomson's discovery of the negatively charged electron had raised theoretical problems for physicists as early as 1897, because atoms as a whole are electrically neutral.
What was Bohr experiment?
In 1913, Niels Bohr proposed a theory for the hydrogen atom, based on quantum theory that some physical quantities only take discrete values. Electrons move around a nucleus, but only in prescribed orbits, and If electrons jump to a lower-energy orbit, the difference is sent out as radiation.
What is Ernest Rutherford experiment?
Ernest Rutherford's most famous experiment is the gold foil experiment. A beam of alpha particles was aimed at a piece of gold foil. Most alpha particles passed through the foil, but a few were scattered backward. This showed that most of the atom is empty space surrounding a tiny nucleus.
How did J.J. Thomson know that the electron was negatively charged?
Thomson discovered the electron by experimenting with a Crookes, or cathode ray, tube. He demonstrated that cathode rays were negatively charged. In addition, he also studied positively charged particles in neon gas.
What did J.J. Thomson discover?
On April 30, 1897, British physicist J.J. Thomson announced his discovery that atoms were made up of smaller components. This finding revolutionized the way scientists thought about the atom and had major ramifications for the field of physics.
What did the gold-foil experiment prove?
The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford's model to make his own.
What experiment led to the discovery of the electron?
Electron was discovered by J. J. Thomson in Cathode Ray Tube (CRT) experiment. The charge of an electron was measured by R. Millikan in Oil drop experiment.
Who did the gold foil experiment?
In 1899 Ernest Rutherford studied the absorption of radioactivity by thin sheets of metal foil and found two components: alpha (a) radiation, which is absorbed by a few thousandths of a centimeter of metal foil, and beta (b) radiation, which can pass through 100 times as much foil before it was absorbed.
When was JJ Thomson's experiment?
The British physicist Joseph John (J. J.) Thomson (1856–1940) performed a series of experiments in 1897 designed to study the nature of electric discharge in a high-vacuum cathode-ray tube, an area being investigated by many scientists at the time.
What is JJ Thomson best known for?
Thomson, in full Sir Joseph John Thomson, (born December 18, 1856, Cheetham Hill, near Manchester, England—died August 30, 1940, Cambridge, Cambridgeshire), English physicist who helped revolutionize the knowledge of atomic structure by his discovery of the electron (1897).
What did the gold-foil experiment prove?
The gold-foil experiment showed that the atom consists of a small, massive, positively charged nucleus with the negatively charged electrons being at a great distance from the centre. Niels Bohr built upon Rutherford's model to make his own.
Who performed the gold-foil experiment?
In 1899 Ernest Rutherford studied the absorption of radioactivity by thin sheets of metal foil and found two components: alpha (a) radiation, which is absorbed by a few thousandths of a centimeter of metal foil, and beta (b) radiation, which can pass through 100 times as much foil before it was absorbed.
Which experiment showed that all atoms contain tiny negatively charged subatomic particles?
Experiments with cathode ray tubes by J. J Thomson have shown that all atoms contain tiny negatively charged subatomic particles, or electrons. The plum pudding model of the atom by Thomson had negatively charged electrons placed inside a "soup" that was positively charged.
Who discovered the electron?
The electron was discovered by J.J. Thomson by cathode ray discharge tube experiment.
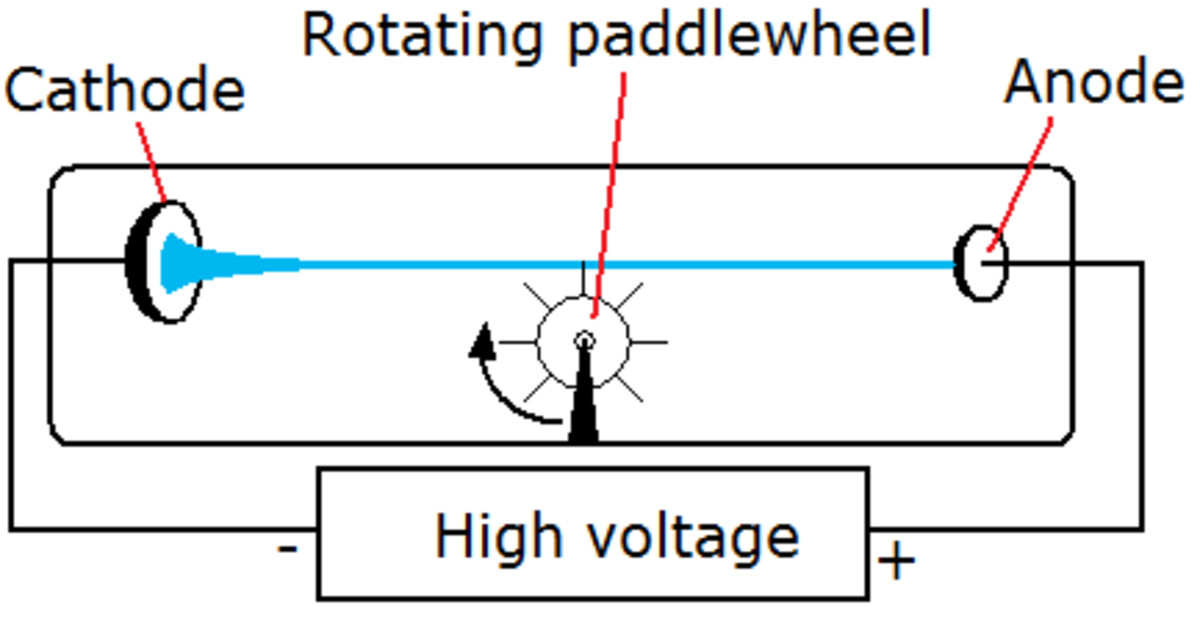
How are electrons flowing towards anode dectected?
The electrons flowing towards anode are dectected by rotation of light paddle wheel kept along their line of motion thu s prooving that electrons are material particles
What would happen if the sign of charges in protons and electrons were reversed in notation or in practice?
If the sign of charges in protons and electrons were reversed in notation or in practice, it would make almost no difference whatsoever.
What is JJ Thompson's famous experiment?
As for practical experiments, you can perform JJ Thompson's famous cathode ray experiment , or read about it. That famous experiment necessarily proves that atoms are surrounded by particles which have negative charge. And I guess, if you perform experiments with static electricity then also you can arrive at the conclusion that electrons have negative charge.
How to prove that an atom is stable?
If you calculate the energy of such systems, you will realise the only way the atom can be stable is when one the electron is opposite in charge to the proton. There are more theoretical ways in which you can prove this by using quantum mechanics which is a bit complicated but if you want to know more then I'd suggest you to do further reading on this.
Which particle is evenly distributed as the repulsion between them due to their similar negative charge?
He said that negatively charged particle named electron is present in an atom as plum in a pudding and it is evenly distributed as the repulsion between them due to their similar negative charge is balanced by the the positive sphere (atom is a positive sphere).
What are the four experiments that led to the discovery of the Cathode Ray Experiment?
4 Cathode Ray Experiment. 1 Physics Experiments 2 Ben Franklin Kite 3 Brownian Movement 4 Cathode Ray Experiment. Like most scientists of that era, he inspired generations of later physicists, from Einstein to Hawking.
How did the magnetic field prove that the negative charge and the ray were inseparable and intertwin?
He found that by applying a magnetic field across the tube, there was no activity recorded by the electrometers and so the charge had been bent away by the magnet. This proved that the negative charge and the ray were inseparable and intertwined.
What is a Cathode Ray Tube?
Even without consciously realizing it, most of us are already aware of what a cathode ray tube is.
What did Thomson find about the cathode rays?
He decided upon the latter and came up with the idea that the cathode rays were made of particles that emanated from within the atoms themselves, a very bold and innovative idea.
What would happen if a glass tube was pumped out of the air?
Physicists in the 19th century found out that if they constructed a glass tube with wires inserted in both ends, and pumped out as much of the air as they could , an electric charge passed across the tube from the wires would create a fluorescent glow. This cathode ray also became known as an ‘electron gun’.
What did Thomson find out about the electron gun?
Thomson had an inkling that the ‘rays’ emitted from the electron gun were inseparable from the latent charge, and decided to try and prove this by using a magnetic field.
What did Bohr and Rutherford prove?
His better-known research proved the existence of negatively charged particles, later called electrons, and earned him a deserved Nobel Prize for physics. This research led to further experiments by Bohr and Rutherford, leading to an understanding of the structure of the atom.
Which scientist put an electric field around a cathode ray tube, which carried a stream of electron?
J.J. Thompson put an electric field around a cathode ray tube, which carried a stream of electrons. The stream of electrons was attracted to the positive pole of the magnetic field. This proved that the electrons carried a negative charge.
What particles were deflected toward the positive plate?
This was Rutherford's other experiment that we mentioned. When radiation was shot through an electric field, the negative particles (beta particles) were deflected toward the positive plate, the positive particles (alpha particles) were deflected toward the negative plate, and the neutral particles (gamma rays) were unaffected by the magnetic field
How much sodium is in a sample of sodium chloride?
two separate 250.0 g pure samples of sodium chloride were analyzed. Both samples were found to contain 39.3% sodium and 60.7% chlorine by mass. Use what you have learning about atomic molecular theory to explain why the same results would be obtained from both samples
What is Chapter 3 of the book Atoms?
Chapter 3: Atoms: The Building Blocks of Matter
What is an atom like?
The few that were deflected, bounced off the nucleus of a gold atom. As Rutherford said, an atom is like a grape hanging in the middle of a cathedral. The nucleus is the grape and the electrons occupy the rest of the interior of the cathedral.
