Cations and Anions
| Formula | Name | Formula | Name |
| Ag+ | Silver | He2+ | Helium |
| Al3+ | Aluminium | Hg2+ | Mercury (II) |
| Ba2+ | Barium | K+ | Potassium |
| Be2+ | Beryllium | Li+ | Lithium |
Full Answer
How can you identify a cation?
Steps of Qualitative Analysis
- If the sample is presented as a solid (salt), it's important to note the shape and color of any crystals.
- Reagents are used to separate cations into groups of related elements.
- Ions in a group are separated from each other. ...
- Separations rely on different characteristics of ions. ...
What makes a cation different from an anion?
What to keep in mind?
- Atoms are unstable under normal conditions.
- To make the atom stable, it should gain or lose one or more valence electrons for it to become an ion.
- The charge of the ion (positive or negative) will depend on the gain or loss.
- A net positive charge ion is called a cation.
- A net negative charge ion is called an anion.
How do cations form?
Summary
- Cations are formed by the loss of one or two electrons from an element.
- Groups 1 and 2 elements form cations.
- Cations are named according to the parent element.
- Cation charges are indicated with a superscript following the chemical symbol.
How do you find the cation and anion?
How to find the cation and anion in a compound?
- Not all compounds have ions. Cation and anion are the exclusive stuff of ionic compounds. ...
- Ionic compounds comprise a metallic and non-metallic element. To find the cation and anion in a compound, we have to first ask if the compound is ionic. ...
- The cation is the metallic component. ...
- The anion is the non-metallic component. ...

What is the formula of cation?
SolutionWrite the formula for aluminum nitride1. Write the symbol and charge of the cation (metal) first and the anion (nonmetal) second.Al3+N3−2. Use a multiplier to make the total charge of the cations and anions equal to each other.total charge of cations = total charge of anions 1(3+) = 1(3-) +3 = -32 more rows•Jul 20, 2022
What is an anion formula?
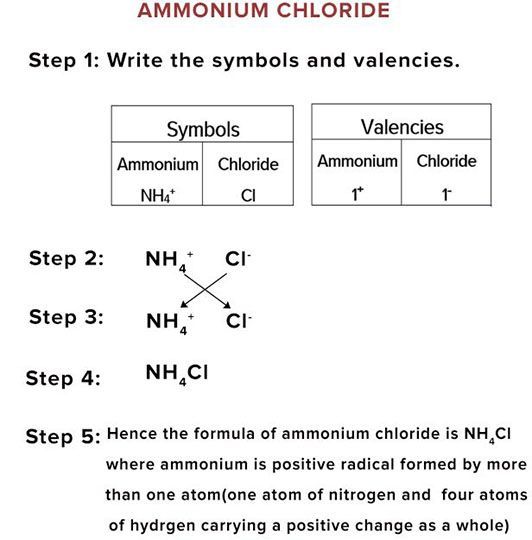
When the cation and/or the anion is a polyatomic ion, parentheses may be used to group the atoms in the ion together to write the formula. For example, the salt ammonium sulfate consists of the cation NH4+ and the sulfate anion SO42-. The formula of the salt is written as (NH4)2SO4.
How do you write a cation and anion formula?
Here are the steps for writing and balancing the formula:Identify the cation ( the portion with a positive charge). ... Identify the anion ( the portion with a negative charge). ... Write the cation first, followed by the anion.Adjust the subscripts of the cation and anion so the net charge is 0.
What is cation in ion?
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Therefore, they possess a net positive charge. Some examples of cations are Calcium (Ca2+), Potassium (K+), hydrogen (H+).
What is the symbol of cation?
IntroductionCationsAnionSymbolNameSymbolH+hydrogen ionH-Li+lithium ionF-Na+sodium ionCl-13 more rows
What is cation and anion example?
When writing the chemical formula of a compound, cation always comes before anion. For example, in NaBr, sodium is the cation, while bromine is the anion.
Is a cation an anion?
Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons).
How do you write formulas?
The correct formulas of the reactants are always written on the left side of the equation. The correct formulas of the products are always written on the right side of the equation. The reactants and products are separated by an arrow pointing toward the products.
Is Na+ an anion or cation?
Neutral sodium atom (Na) becomes sodium cation (Na+) by releasing an electron.
Why is it called a cation?
Some elements lose one or more electrons in forming ions. These ions are known as "cations" because they are positively charged and migrate toward the negative electrode (cathode) in an electrical field.
What anion means?
a negatively charged ionDefinition of anion : the ion in an electrolyzed solution that migrates to the anode broadly : a negatively charged ion.
Is hydrogen a cation?
Hydrogen forms the only cation that has no electrons, but even cations that (unlike hydrogen) still retain one or more electrons are still smaller than the neutral atoms or molecules from which they are derived.
What anion means?
a negatively charged ionDefinition of anion : the ion in an electrolyzed solution that migrates to the anode broadly : a negatively charged ion.
What is an anion in chemistry?
anion, atom or group of atoms carrying a negative electric charge.
Is nacl an anion?
In table salt, sodium chloride, sodium is the cation (Na+) and chloride is the anion (Cl-).
What is difference between cation and anion?
⚡️ Quick summary. Cations are positively-charged ions (atoms or groups of atoms that have more protons than electrons due to having lost one or more electrons). Anions are negatively-charged ions (meaning they have more electrons than protons due to having gained one or more electrons).
What is a cation?
A cation is a positively charged ion. A cation can be composed of: ⚛ one atom (monoatamic cation) ⚛ more than one atom (polyatomic cation) The formula of a monoatomic (1) cation is made up of two parts: Formula of Monoatomic Cation. symbol of element. charge as a superscript (2) to the right of the symbol. E.
What is the name of H+?
That is, the name of H + is hydrogen (1+) or hydron. (4) Heteropolyatomic cations are usually named either substitutively or additively. Substitutive names do not require a charge number because the name itself implies the charge. Azanium is the name for NH 4+ using substitutive nomenclature.
What is a cation?
Cations are ions which have a positive electrical charge. A cation has fewer electrons than protons. An ion may consist of a single atom of an element ( a monatomic ion or monatomic cation or anion) or of several atoms that are bonded together ( a polyatomic ion or polyatomic cation or anion ).
Why are cations attracted to anions?
Because of their net electrical charge, cations are repelled by other cations and are attracted to anions. This is a table listing the name, formula, and charge of common cations. Alternate names are given for some cations.
Which element is always +2 when it forms cations?
The alkaline earth metals in group 2 are always +2 when they form cations. Aluminum and the elements in group 3 are always +3 when they form cations. Zinc and cadmium always form +2 cations. Although silver can form both +1 and +2 cations, the +2 is so rare that we usually name Ag + as silver ion, not silver (I) ion.
What does the Roman numeral mean in monatomic cations?
The names of monatomic cations always start with the name of the metal, sometimes followed by a Roman numeral to indicate the charge of the ion. For example, Cu + is copper (I), and Cu 2+ is copper (II). The Roman numeral in each name represents the charge on the ion and allows us to distinguish between more than one possible charge. Notice that there is no space between the end of the name of the metal and the parentheses with the Roman numeral.
How do nonmetals and metallic elements form ionic bonds?
Thus, when a metallic element and a nonmetallic element combine, the nonmetallic atoms often pull one or more electrons far enough away from the metallic atoms to form ions. The positive cations and the negative anions then attract each other to form ionic bonds.
What metals lose electrons to form cations?
The metallic elements in groups other than 1, 2, or 3 also lose electrons to form cations, but they do so in less easily predicted ways. It will be useful to memorize some of the charges for these metals. Ask your instructor which ones you will be expected to know. To answer the questions in this text, you will need to know that iron atoms form both Fe 2+ and Fe 3+, copper atoms form Cu + and Cu 2+, zinc atoms form Zn 2+, cadmium atoms form Cd 2+, and silver atoms form Ag + . The image below summarizes the charges of the ions that you should know at this stage.
Why do we put the Roman numeral in manganese?
We know to put the Roman numeral in the name because manganese is not on our list of metals with only one charge. Polyatomic Cation Names. There is only one common polyatomic ion. Its formula is NH 4+, and its name is ammonium.
Is it wrong to put a Roman numeral after an element?
If the atoms of an element always have the same charge, the Roman numeral is unnecessary (and considered to be incorrect). For example, all cations formed from sodium atoms have a +1 charge, so Na + is named sodium ion, without the Roman numeral for the charge. The following elements have only one possible charge, so it would be incorrect to put a Roman numeral after their name.
What is an ion?
Any atom or molecule with a net charge, either positive or negative, is known as an ion. Ions are formed when the number of protons in an atom does not equal the number of electrons. If more protons are present, the ion is positive and is known as a cation; if more electrons are present, the ion is negative and referred to as an anion. An ion consisting of a single atom is a monatomic ion; an ion consisting of two or more atoms is referred to as a polyatomic ion.
How are ionic compounds named?
Ionic compounds are named after their cation and anion. Any atom or molecule with a net charge, either positive or negative, is known as an ion. Ions are formed when the number of protons in an atom does not equal the number of electrons. If more protons are present, the ion is positive and is known as a cation; if more electrons are present, ...
What is the name of the ion that is positive?
If more protons are present, the ion is positive and is known as a cation ; if more electrons are present, the ion is negative and referred to as an anion. • When ionic solids are dissolved in water or a solvent they form a solution of free cations and anions that can conduct electricity, this is known as an electrolyte. Key Terms.
What is an ion that has two or more atoms?
An ion consisting of a single atom is a monatomic ion; an ion consisting of two or more atoms is referred to as a polyatomic ion.
What is a substance that is dissolved in a liquid solvent to create a solution?
Solute: Any substance that is dissolved in a liquid solvent to create a solution.
What are cations?
Cations are positively charged ions. They are formed when a metal loses its electrons. They lose one or more than one electron and do not lose any protons. Therefore, they possess a net positive charge. Some examples of cations are Calcium (Ca 2+ ), Potassium (K + ), hydrogen (H + ).
What is the difference between an anion and a cation?
An anion may be defined as an atom or molecule that is negatively charged. A cation may be defined as an atom or molecule that is positively charged. Anions and cations are both ions. They have an opposite electrical charge, therefore they get attracted to each other.
How do cations and anions differ?
They have an opposite electrical charge, therefore they get attracted to each other. Cation repels other cation whereas anion repels another anion. The number of protons is more than the number of electrons in a cation whereas the number of electrons is more than the number of protons in an anion.
What are ions with negative charges called?
The ions with a negative charge are called anions and the ones with a positive charge are called cations. Since both of them have charges of opposing qualities, they get attracted to one another and thereby forming an ionic bond between them.
What are ions in chemistry?
Ions which are a part of the science subject Chemistry forms from atoms and electrons that have either gained or lost their weight by the removal or adding of one or more valence electrons which would create either positive or a negative charge. The ions with a negative charge are called anions and the ones with a positive charge are called cations.
Which anions have a net negative charge?
Therefore, they possess a net negative charge. Some examples of anions are Iodide (I – ), chlorine (Cl – ), hydroxide (OH – ). When sodium a cation is depicted as Na +, the plus charge indicator shows that it has one electron less than the total number of protons.
Is Cl an anion?
Cl- is an example of an anion. A Cl- atom is a chlorine atom that has gained one electron and hence has a negative charge of -1 since it has a full outer shell.
What is the ratio of cation and anion?
If the charges of the cation and anion are equal (e.g., +1/-1, +2/-2, +3/-3), then combine the cation and anion in a 1:1 ratio . An example is potassium chloride, KCl. Potassium (K +) has a 1- charge, while chlorine (Cl -) has a 1- charge. Note that you do not ever write a subscript of 1.
What is the first ion in an ionic compound?
The positive ion, called a cation , is listed first in an ionic compound formula, followed by the negative ion, called an anion. A balanced formula has a neutral electrical charge or net charge of zero.
Which ion is the least electronegative?
Identify the cation ( the portion with a positive charge). It is the least electronegative (most electropositive) ion. Cations include metals and they are often located on the left-hand side of the periodic table. Identify the anion ( the portion with a negative charge). It is the most electronegative ion. Anions include halogens and nonmetals.
Is there a subscript for carbonate anion?
The carbonate anion (CO 3-2) has a 2- charge, so there is no additional subscript. If you need to add a subscript to a polyatomic ion, enclose it in parentheses so it is clear the subscript applies to the entire ion and not to an individual atom. An example is aluminum sulfate, Al 2 (SO 4) 3.