What is the molecular geometry of permanganate?
The ion has tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media. The exact chemical reaction is dependent upon the organic contaminants present and the oxidant utilized.
How do you make permanganates?
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide : It may also be produced by the disproportionation of manganates, with manganese dioxide as a side-product: 4 ).
What can a permanganate do to an amine?
A permanganate can oxidize an amine to a nitro compound, an alcohol to a ketone, an aldehyde to a carboxylic acid, a terminal alkene to a carboxylic acid, oxalic acid to carbon dioxide, and an alkene to a diol. This list is not exhaustive. In alkene oxidations one intermediate is a cyclic Mn (V) species :
Can acetic acid explode with permanganates?
Acetic acid or acetic anhydride can explode with permanganates if not kept cold, [Von Schwartz 1918. p. 34]. Explosions can occur when permanganates, treated with sulfuric acid come in contact with benzene, carbon disulfide, diethyl ether, ethyl alcohol, petroleum, or organic matter.

What is PB mno42?
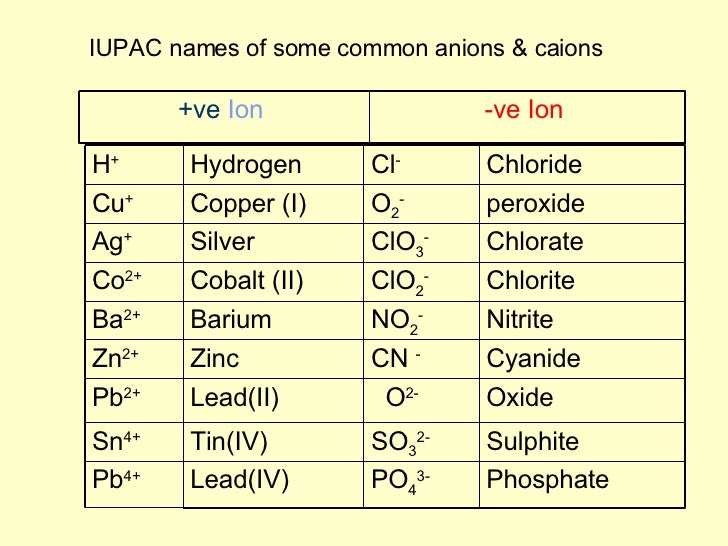
Lead(II) Permanganate. Pb(MnO4)2. Lead(II) Chlorate.
What is the name for co MnO4 2?
Cobalt(II) Permanganate Co(MnO4)2 Molecular Weight -- EndMemo.
How do you write the formula for nickel II permanganate?
Nickel(II) Permanganate Ni(MnO4)2 Molecular Weight -- EndMemo.
What is the name of HG MnO4 2?
Mercury(II) Permanganate Hg(MnO4)2 Molecular Weight -- EndMemo.
What is mn04?
A permanganate is the general name for a chemical compound containing the manganate(VII) ion, MnO − 4. , the conjugate base of permanganic acid. Because the manganese atom is in the +7 oxidation state, the permanganate(VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry.
What is Li2Cr2O7?
Lithium dichromate | Li2Cr2O7 - PubChem.
What ion is sn4+?
Tin(4+)Tin(4+) is a monoatomic tetracation and an elemental tin.
What is the formula for Ag+ and s2?
Silver sulfideNamesChemical formulaAg2SMolar mass247.80 g·mol−1AppearanceGrayish-blackish crystalOdorOdorless41 more rows
What is the name of Cu2Cr2O7?
Copper(I) Dichromate Cu2Cr2O7 Molecular Weight -- EndMemo.
What is the Colour of mno4 2?
The colour and magnetic nature of manganate ion (MnO42-) is - India Site. Explanation: The colour and magnetic nature of manganate ion (MnO42-) is green, paramagnetic.
What is the charge of each mercury ion in hg2 2+?
2Dimercury(2+) is a mercury cation....3.1Computed Properties.Property NameProperty ValueReferenceFormal Charge2Computed by PubChemComplexity0Computed by Cactvs 3.4.6.11 (PubChem release 2019.06.18)15 more rows
What is the structure of mno4?
MnO4– (permanganate) has one manganese atom and four oxygen atoms. In the lewis structure of MnO4–, there is one single bond and three double bonds around the manganese atom, with four oxygen atoms attached to it.
What type of compound is Mg MnO4 2?
Magnesium Permanganate Mg(MnO4)2 Molecular Weight -- EndMemo.
What type of compound is Mg3N2?
Magnesium nitride, which possesses the chemical formula Mg3N2, is an inorganic compound of magnesium and nitrogen.
What is the name of kmno4?
Potassium manganate(VII)Potassium permanganate / IUPAC ID
What type of compound is P2Br4?
Diphosphorus Tetrabromide P2Br4 Molecular Weight -- EndMemo.
3.2 Experimental Properties
Generally purplish colored. Soluble in water. Noncombustible, but accelerate the burning of combustible material. If the combustible material is finely divided, the mixture may be explosive. May spontaneously ignite in contact with liquid combustible materials. Contact with sulfuric acid may cause fire or explosion.
6.1 MeSH Pharmacological Classification
Electron-accepting molecules in chemical reactions in which electrons are transferred from one molecule to another (OXIDATION-REDUCTION). (See all compounds classified as Oxidants .)
7.1 Hazards Identification
Excerpt from ERG Guide 140 [Oxidizers]: Inhalation, ingestion or contact (skin, eyes) with vapors or substance may cause severe injury, burns or death. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may cause pollution. (ERG, 2016)
7.2 First Aid Measures
Excerpt from ERG Guide 140 [Oxidizers]: Ensure that medical personnel are aware of the material (s) involved and take precautions to protect themselves. Move victim to fresh air. Call 911 or emergency medical service. Give artificial respiration if victim is not breathing. Administer oxygen if breathing is difficult.
7.3 Fire Fighting
Excerpt from ERG Guide 140 [Oxidizers]: SMALL FIRE: Use water. Do not use dry chemicals or foams. CO2 or Halon ® may provide limited control. LARGE FIRE: Flood fire area with water from a distance. Do not move cargo or vehicle if cargo has been exposed to heat. Move containers from fire area if you can do it without risk.
7.4 Accidental Release Measures
Excerpt from ERG Guide 140 [Oxidizers]: As an immediate precautionary measure, isolate spill or leak area in all directions for at least 50 meters (150 feet) for liquids and at least 25 meters (75 feet) for solids. LARGE SPILL: Consider initial downwind evacuation for at least 100 meters (330 feet).
7.5 Handling and Storage
Excerpt from ERG Guide 140 [Oxidizers]: Keep combustibles (wood, paper, oil, etc.) away from spilled material. Do not touch damaged containers or spilled material unless wearing appropriate protective clothing. Stop leak if you can do it without risk. Do not get water inside containers.
What is the oxidation state of permanganate?
In an acidic solution, permanganate (VII) is reduced to the pale pink +2 oxidation state of the manganese (II) (Mn 2+) ion.
How are permanganates produced?
Permanganates can be produced by oxidation of manganese compounds such as manganese chloride or manganese sulfate by strong oxidizing agents, for instance, sodium hypochlorite or lead dioxide :
Why is permanganate purple?
They have a deep purple colour, due to a charge transfer transition. Permanganate (VII) is a strong oxidizer, and similar to perchlorate. It is therefore in common use in qualitative analysis that involves redox reactions ( permanganometry ).
What intermediate is used in alkene oxidation?
In alkene oxidations one intermediate is a cyclic Mn (V) species :
Is manganese oxidized?
Because manganese is in the +7 oxidation state, the permanganate (VII) ion is a strong oxidizing agent. The ion has tetrahedral geometry. Permanganate solutions are purple in color and are stable in neutral or slightly alkaline media.
Can permanganate oxidize alcohol?
A permanganate can oxidize an amine to a nitro compound, an alcohol to a ketone, an aldehyde to a carboxylic acid, a terminal alkene to a carboxylic acid, oxalic acid to carbon dioxide, and an alkene to a diol. This list is not exhaustive. In alkene oxidations one intermediate is a cyclic Mn (V) species :
Is permanganate a disinfectant?
Besides this, it is stable. It is a useful reagent, but it is not very selective with organic compounds. Potassium permanganate is used as a disinfectant and water treatment additive in aquaculture.
