
What are the four requirements of basic combustion?
- The inlet valve should be as large as possible to achieve a high volumetric efficiency.
- The length the flame has to travel should be as short as possible to avoid detonation of the charge. ...
- Hot spots that can cause pre-ignition should be avoided by cooling and the location of the spark plug and the exhaust valve. ...
What does the combustion equation Mean?
The word equation for the combustion of butane is, butane + oxygen → carbon dioxide + water. Like all combustion reactions, oxygen will always be one of the and be on the left of the equation. Most combustion reactions produce carbon dioxide and water, so these chemicals are written as the on the right of the equation.
What is the general equation for complete combustion?
The general equation for a complete combustion reaction is: Fuel + O2 → CO2 + H2O. The burning of charcoal is a combustion reaction. Is the combustion of butane exothermic or endothermic? Butane releases its chemical energy by undergoing hydrocarbon combustion.
What is the chemical formula of combustion?
The general form of a combustion reaction can be represented by the reaction between a hydrocarbon and oxygen, which yields carbon dioxide and water: hydrocarbon + O 2 → CO 2 + H 2 O . In addition to heat, it’s also common (although not necessary) for a combustion reaction to release light and produce a flame.
What is combustion in chemistry?
combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame.
What is combustion Class 10th?
Combustion Meaning Combustion refers to the process where a substance burns in the presence of Oxygen, giving off heat and light in the process.
What is example of combustion reaction?
Examples of Combustion Reactions Imagine the wood burning in a fireplace. The wood is fuel which continuously reacts with oxygen in the air in a combustion reaction. The fire produced is energy being released in a long exothermic reaction, while the smoke you see is carbon dioxide.
What are 5 examples of combustion reactions?
Examples of Combustion ReactionsCombustion of methane. CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)Burning of naphthalene. ... Combustion of ethane. ... Combustion of butane (commonly found in lighters) ... Combustion of methanol (also known as wood alcohol) ... Combustion of propane (used in gas grills, fireplaces, and some cookstoves)
What are the 3 types of combustion?
Types of CombustionRapid Combustion,Spontaneous Combustion, and.Explosive Combustion.
What is combustion answer?
Combustion is a chemical process or a reaction between Fuel (Hydrocarbon) and Oxygen. When fuel and oxygen react it releases the heat and light energy. Heat and light energy then result in the flame. So, the formula for Combustion reaction is Hydrobcarbon + Oxygen = Heat energy.
What is complete combustion?
Complete combustion happens when there is a good supply of air. Carbon and hydrogen atoms in the hydrocarbon fuel react with oxygen in an exothermic reaction: carbon dioxide and water are produced. energy is given out.
Which type of reaction is combustion?
exothermic redox reactionsCombustion reactions are generally highly exothermic redox reactions between an oxidant and a fuel. The product formed in a combustion reaction is usually the oxidised fuel (which is mostly liberated in the gaseous state).
What are the two types of combustion?
Combustion is applicable to two types of fire: Flaming combustion and smoldering combustion [13].
What is combustion used for?
Combustion systems utilize the energy of chemical compounds released during this reactive process for transportation, to generate electric power, or to provide heat for various applications. Chemistry and combustion are interlinked in several ways.
How is heat of combustion used?
Heats of combustion are used as a basis for comparing the heating value of fuels, since the fuel that produces the greater amount of heat for a given cost is the more economic. Heats of combustion are also used in comparing the stabilities of chemical compounds.
What is combustion and example?
Definition of combustion The chemical process in which a substance reacts with oxygen to give off heat is called combustion. Examples are ethane, wood, propane.
What is combustion and types?
Combustion is the act of burning, in which fuel, heat and oxygen release energy. There are several types of combustion, such as internal combustion, diesel combustion, low temperature combustion and other novel forms.
What is combustion of fuel?
Combustion is a complex series of chemical reactions, but from a physical standpoint may be described as the rapid combination of oxygen with a fuel, such as natural gas or wood, resulting in the release of heat. Most fuels contain carbon and hydrogen, and the oxygen usually comes from air.
What is combustion in the carbon cycle?
Carbon Cycle - Combustion/Metabolism Reaction: Combustion occurs when any organic material is reacted (burned) in the presence of oxygen to give off the products of carbon dioxide and water and ENERGY. The organic material can be any fossil fuel such as natural gas (methane), oil, or coal.
What is combustion reaction?
Combustion Reaction. Combustion reaction refers to an exothermic chemical reaction that has high-temperature. It happens between a fuel (the reductant) and an oxidant, usually atmospheric oxygen that produces oxidized, often gaseous products, resulting in a mixture of smoke. Combustion reaction in a fire produces a flame, ...
What is the energy required to kick start a combustion?
To kick-start the combustion, energy is required to force dioxygen into a spin-paired state, or singlet oxygen. This intermediate is extremely reactive. It supplies the energy as heat, and the reaction then produces additional heat, which allows it to continue.
What is the reaction that occurs when the combustion is quenched by a heat sink?
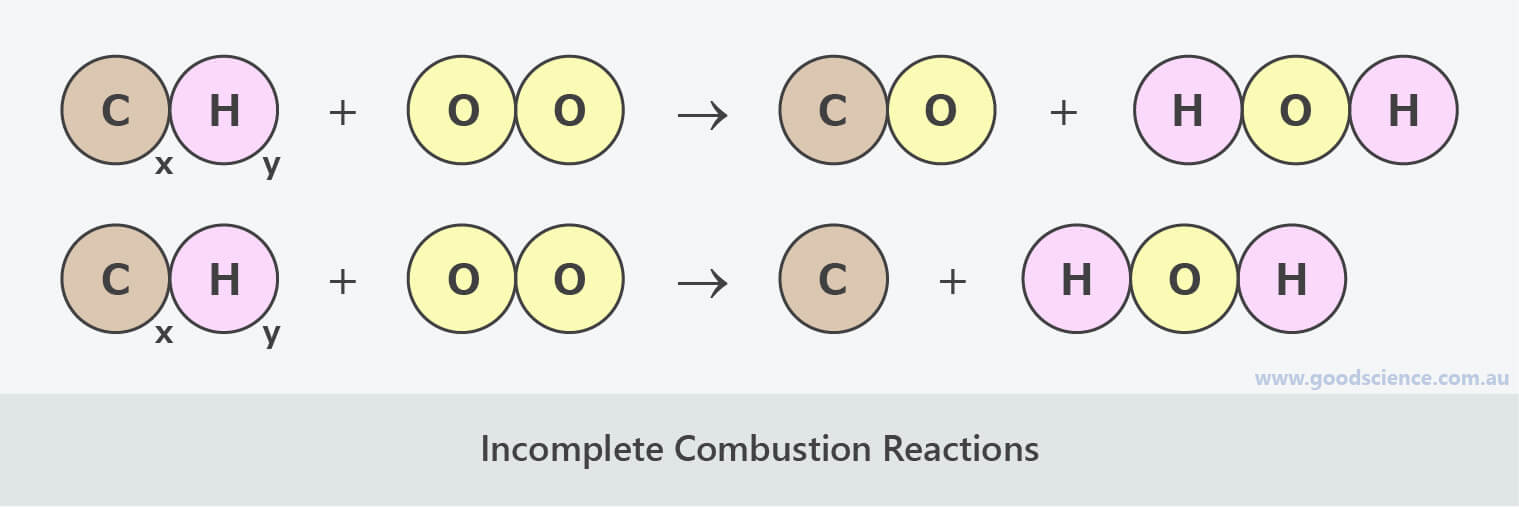
Incomplete Combustion Reaction. This type of reaction occurs when there is short of oxygen to allow the fuel to react completely to produce carbon dioxide and water. It also occurs when the combustion is quenched by a heat sink, such as a solid surface or flame trap. Carbon, carbon monoxide, and/or hydroxide is produced instead of carbon dioxide.
What is rapid reaction?
Rapid Reaction. As the name suggests it is a fast combustion reaction, with the release of large amounts of heat and light energy, which often results in a flame. For example, Fire which releases both heat and light.
What is the reaction of hydrogen atoms to oxygen?
In the case of hydrocarbons, the hydrogen atom abstraction (not proton abstraction) initiates the reaction from the fuel to oxygen, to give a hydroperoxide radical (HOO). This HOO reacts further to give hydroperoxides, which break up to give hydroxyl radicals.
What happens when a hydrocarbon burns in oxygen?
When a hydrocarbon burns in the presence of oxygen, the reaction will primarily yield carbon dioxide and water. When elements burn, the products are primarily the most common oxides.
What is spontaneous reaction?
Spontaneous Reaction. It is a type of combustion, which occurs by self-heating, followed by thermal runaway and finally, ignition. For example, phosphorus burns at room temperature without the application of heat.
When is combustion complete?
For example, if methane reacts with oxygen and produces only carbon dioxide and water, the process is complete combustion.
How does combustion occur?
Combustion occurs when fuel and an oxidant react to form oxidized products. Typically, energy must be supplied to initiate the reaction. Once combustion starts, the released heat can make combustion self-sustaining.
What are some examples of combustion reactions?
A simple example of a combustion reaction is the reaction between hydrogen gas and oxygen gas to produce water vapor: A more familiar type of combustion reaction is the combustion of methane (a hydrocarbon) to produce carbon dioxide and water: which leads to one general form of a combustion reaction:
Why does combustion release heat?
The reason combustion releases heat is because the double bond between oxygen atoms in O 2 is weaker than the single bonds or other double bonds. So, although energy is absorbed in the reaction, it is released when the stronger bonds are formed to make carbon dioxide (CO 2) and water (H 2 O). While the fuel plays a role in the energy ...
What are the oxidants in combustion?
These include pure oxygen and also chlorine, fluorine, nitrous oxide, nitric acid, and chlorine trifluoride.
What is the name of the chemical reaction that occurs between a fuel and an oxidizing agent that produces energy?
Combustion is a chemical reaction that occurs between a fuel and an oxidizing agent that produces energy, usually in the form of heat and light. Combustion is considered an exergonic or exothermic chemical reaction. It is also known as burning. Combustion is considered to be one of the first chemical reactions intentionally controlled by humans.
What happens to fuels before combustion?
Incomplete oxidation of a fuel may also occur. It also results when pyrolysis occurs before combustion, as is the case with most fuels. In pyrolysis, organic matter undergoes thermal decomposition at high temperatures without reacting with oxygen.
What is combustion in science?
What is Combustion? The Fuse School - Global Education (YouTube) Fire is a chemical chain reaction which takes place with the evolution of heat and light. In order for a fire to take place there are 3 main ingredients that must be present: Oxygen, Heat and Fuel.
Where does the initial energy come from in a car?
Other sources of initial energy can come from the Sun, matches, friction, etc. The combustion reaction itself is quite exothermic.
What is combustion based on?
Combustion encompasses a great variety of phenomena with wide application in industry, the sciences, professions, and the home, and the application is based on knowledge of physics, chemistry, and mechanics; their interrelationship becomes particularly evident in treating flame propagation.
What are some examples of combustion reactions?
A familiar example of a combustion reaction is a lighted match. When a match is struck, friction heats the head to a temperature at which the chemicals react and generate more heat than can escape into the air, and they burn with a flame. If a wind blows away the heat or the chemicals are moist and friction does not raise the temperature sufficiently, the match goes out. Properly ignited, the heat from the flame raises the temperature of a nearby layer of the matchstick and of oxygen in the air adjacent to it, and the wood and oxygen react in a combustion reaction. When equilibrium between the total heat energies of the reactants and the total heat energies of the products (including the actual heat and light emitted) is reached, combustion stops. Flames have a definable composition and a complex structure; they are said to be multiform and are capable of existing at quite low temperatures, as well as at extremely high temperatures. The emission of light in the flame results from the presence of excited particles and, usually, of charged atoms and molecules and of electrons.
What is the most important chemical reaction?
In general terms, combustion is one of the most important of chemical reactions and may be considered a culminating step in the oxidation of certain kinds of substances.
What is the composition of a flame?
Flames have a definable composition and a complex structure; they are said to be multiform and are capable of existing at quite low temperatures, as well as at extremely high temperatures. The emission of light in the flame results from the presence of excited particles and, usually, of charged atoms and molecules and of electrons.
What is the chemistry of lighting a match?
Learn about the chemistry of lighting a match. Combustion, a chemical reaction between substances, usually including oxygen and usually accompanied by the generation of heat and light in the form of flame. The rate or speed at which the reactants combine is high, in part because of the nature of the chemical reaction itself ...
How to calculate heat of combustion?
To calculate the heat of combustion, use Hess’s law, which states that the enthalpies of the products and the reactants are the same. Start by writing the balanced equation of combustion of the substance. Then, add the enthalpies of formation for the reactions.
What is the heat of combustion?
The Heat of Combustion of a substance is defined as the amount of energy in the form of heat is liberated when an amount of the substance undergoes combustion. Steps.
How to find the amount of a substance burned?
Find the amount of substance burned by subtracting the final mass from the initial mass of the substance in g.
How to measure the mass of a candle?
Measure the mass of the candle and note it in g. Light the substance. When the temperature of the water reaches 40 degrees Centigrade , blow out the substance. Measure the mass of the candle after burning and note it. ...
Meaning of Combustion Reaction
Types of Combustion Reaction
- Complete Combustion Reaction
In this process, the reactant burns in oxygen and produces a limited number of products. When a hydrocarbon burns in the presence of oxygen, the reaction will primarily yield carbon dioxide and water. When elements burn, the products are primarily the most common oxides. - Incomplete Combustion Reaction
This type of reaction occurs when there is short of oxygen to allow the fuel to react completely to produce carbon dioxide and water. It also occurs when the combustion is quenched by a heat sink, such as a solid surface or flame trap. Carbon, carbon monoxide, and/or hydroxide is produc…
Micro-Gravity
- It refers to a “Low” gravitational state to such a level that the influence of buoyancy on physical processes is comparatively small relative to other flow processes that are present at normal gravity. However, in this kind of environment, the thermal and flow transport dynamics usually behaves quite differently than in normal gravity conditions (e.g., a candle’s flame takes the shap…
Reaction Mechanism
- The entire combustion reaction-taking place in oxygen is a chain reaction in which many distinct radical intermediates participate. The great energy required for initiation is explained by the unusual structure of the dioxygen molecule. The less-energy configuration of the dioxygen molecule is a stable, relatively unreactive diradical in a triplet spin state. Most of the fuels are us…
Examples of Combustion Reaction
- Burning of any kind of Wood or Coal to heat your home.
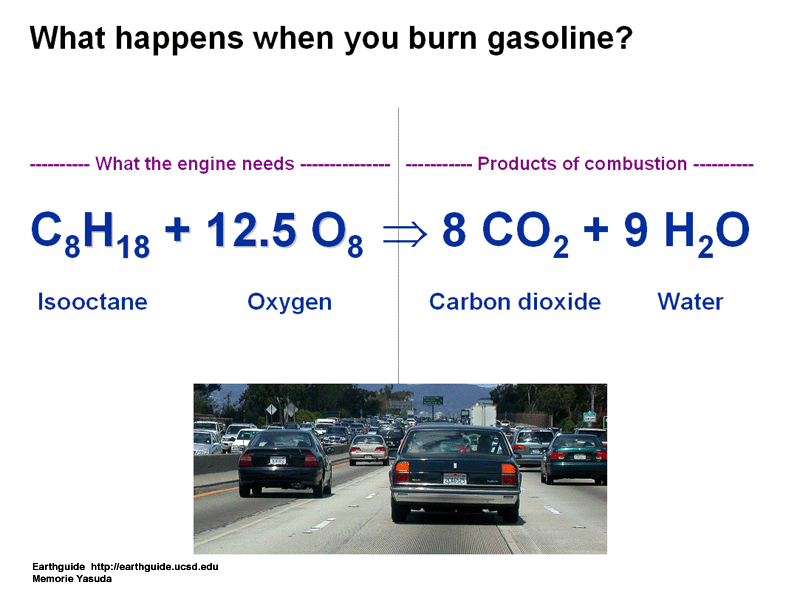
- Car and buses burn petrol or diesel to run.
- Natural Gas or LPG is in use on your stovetop. Combustion of these gases helps in cooking.
- For the production of energy in thermal power plants.
Solved Questions For You
- Q.1. How do you know when a combustion reaction is complete? Ans:Combustion requires three things to occur: fuel (hydrocarbons), oxygen (from the air), and a catalytic spark. Complete combustion on its completion will produce only carbon dioxide and water as the products and nothing will be leftover. However, incomplete combustion will produce other byproducts like car…