/BalanceEquations3-56a132763df78cf77268517f.png)
What is the formula for compound rust?
Rust is a hydrated form of a compound known as iron(III)oxide. Rust is a general term that defines a series of iron oxides (red oxides). The process is formed by the reaction of iron with oxygen in the presence of air moisture or water. The rust formula is approximately Fe 2 O 3 • 32H 2 O, however, the exact amount of water in the formula is ...
What is the word equation for rust?
Key Takeaways: How Rust Works
- Rust is the common name of the chemical called iron oxide. Technically, it's iron oxide hydrate, because pure iron oxide isn't rust.
- Rust forms when iron or its alloys are exposed to moist air. ...
- The familiar red form of rust is (Fe 2 O 3 ), but iron has other oxidation states, so it can form other colors of rust.
What is the molecular formula of rust?
What Is Rust? Rust (Fe 2 O 3 •nH 2 O) is an iron oxide — usually reddish-brown — and is the common term for corrosion of iron and its alloys (steel, cast iron, etc.). Corrosion is described as the destruction of materials by chemical reactions with different substances in the environment. There are three types of corrosion: Chemical; Electrochemical
What is the formula of rusting?
The rusting of the iron formula is represented by 4Fe + 3O2 + 6H2O → 4Fe (OH)3. Rusting can be prevented by galvanization, painting, and application of grease. Q1.

What Is Rust?
Rust ( Fe2O3•nH2O) is an iron oxide — usually reddish-brown — and is the common term for corrosion of iron and its alloys (steel, cast iron, etc.). Corrosion is described as the destruction of materials by chemical reactions with different substances in the environment.
How to make iron rust?
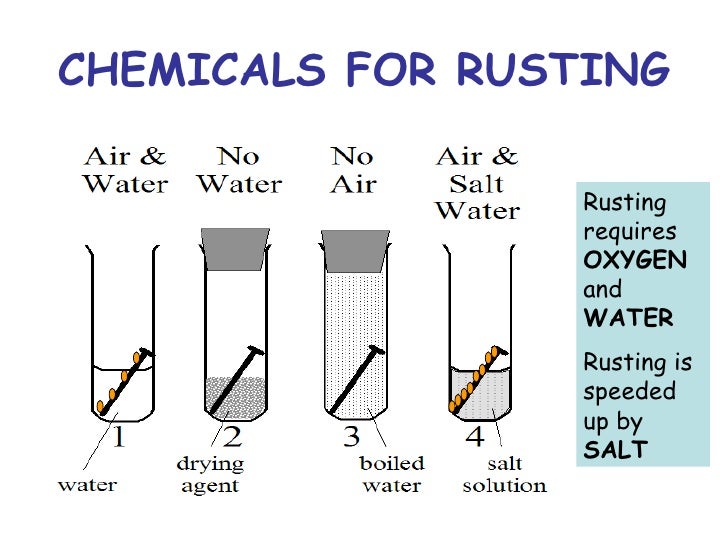
The best way to understand the process is by seeing it with your own eyes. Fortunately for our experiment (and unfortunately in any other terms), iron rusts fairly quickly, so you can observe that process in a matter of days. Here’s what you’ll need for the project: 1 Three empty jars 2 Three iron nails 3 Distilled water 4 Boiled distilled water 5 Vegetable Oil 6 Anhydrous calcium chloride 7 A jar lid
How to understand iron rust?
The best way to understand the process is by seeing it with your own eyes. Fortunately for our experiment (and unfortunately in any other terms), iron rusts fairly quickly, so you can observe that process in a matter of days. Here’s what you’ll need for the project: Three empty jars. Three iron nails.
Is corrosion a problem?
Therefore, corrosion is a huge problem and may lead to machine failures and other stumbling blocks that inevitably cause financial losses. Fortunately, there are ways to prevent metals from rusting and corroding. That is done by creating a barrier between the materials we want to protect and the environment.
Does rusting affect iron?
Rusting is an immensely common process that, unfortunately, affects us negatively. It is quite straightforward — iron or its alloys react with water and air and consequently get coated with rust. That weakens the metal, and if given enough time, any iron object can turn entirely to rust.
Does iron rust in jars?
When you come back, you’ll see that the iron nail in the first jar has started to rust. The nails in the other two, however, are perfectly fine. That proves that in order for rusting to occur, iron has to react with both water and air. If you remove one of the two reactants, no rust will form on the metal’s surface.
What is rusting iron?
Rusting of iron refers to the formation of rust, a mixture of iron oxides, on the surface of iron objects or structures. This rust is formed from a redox reaction between oxygen and iron in an environment containing water (such as air containing high levels of moisture). The rusting of iron is characterized by the formation of a layer of a red, ...
How is rusting iron controlled?
Therefore, the rusting of iron can be controlled by limiting the amount of oxygen and water surrounding the metal.
Why is Rusting an Undesirable Phenomenon?
Rusting causes iron to become flaky and weak, degrading its strength, appearance and permeability. Rusted iron does not hold the desirable properties of iron. The rusting of iron can lead to damage to automobiles, railings, grills, and many other iron structures.
How can Rusting be Prevented?
Iron and its alloys are widely used in the construction of many structures and in many machines and objects. Therefore, the prevention of the corrosion of iron is very important. Some preventive methods are listed below.
What happens when iron rusts?
The reaction of the rusting of iron involves an increase in the oxidation state of iron, accompanied by a loss of electrons. Rust is mostly made up of two different oxides of iron that vary in the oxidation state of the iron atom. These oxides are:
How are iron hydroxides formed?
The hydroxides of iron are also formed from the direct reaction between the iron cations and hydroxide ions. O2 + H2O + 4e– → 4OH–. Fe2+ + 2OH– → Fe (OH)2. Fe3+ + 3OH– → Fe (OH)3. The resulting hydroxides of iron now undergo dehydration to yield the iron oxides that constitute rust.
What causes iron to rust faster?
Acid: if the pH of the environment surrounding the metal is low, the rusting process is quickened. The rusting of iron speeds up when it is exposed to acid rains. Higher pH inhibits the corrosion of iron.