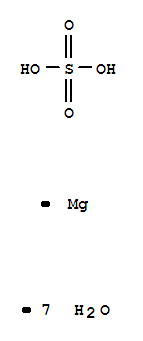
What is the correct formula for magnesium sulfate?
The chemical formula for magnesium sulfate is MgSO4. A magnesium sulfate molecule is composed of a magnesium atom, a sulfur atom, and four oxygen atoms. Below is a diagram of what magnesium sulfate looks like at the molecular level.
What are the adverse effects of magnesium sulfate?
Magnesium sulfate Side effects (9) Diaphoresis, diarrhea, depressed reflexes, facial flushing, hypotension, hypothermia, circulatory collapse, reduced heart rate, respiratory depression. Magnesium sulfate Interactions
Is magnesium sulfate good for weight loss?
This compound also displays analgesic effects and may be used in general anesthesia and sedation as an adjuvant drug , or a drug that has pain-relieving properties although its primary use is not pain relief. As mentioned earlier, Epsom salt, aka magnesium sulfate, is promoted as a natural weight loss aid.
How to write the formula for magnesium sulfate?
magnesium sulphate heptahydrate ChEBI MgSO 4 ·7H 2 O

What is MgSO4 * 7H2O?
Magnesium sulfate heptahydrate Synonym(s): Epsom salts. Linear Formula: MgSO4 · 7H2O.
What is the of water in the hydrate MgSO4 * 7H2O?
The % of H2O in MgSO4. 7H2O=51. 2.
Is MgSO4 7H2O a salt?
Magnesium sulfate, chemical formula MgO4S, and CAS No. 7487-88-9, is an inorganic salt chemical compound containing magnesium, sulfur and oxygen. It is often encountered as the heptahydrate sulfate mineral epsomite MgSO4·7H2O, commonly called Epsom salt.
How is MgSO4 7H2O formed?
Magnesium sulfate-7-water, MgSO4. 7H2O, crystals are obtained by evaporation. They may be recrystallised to increase purity. You can make magnesium sulfate-7-water in the laboratory by reacting magnesium oxide with dilute sulfuric acid.
How do you calculate the percentage of water of crystallization in MgSO4 7H2O?
Percent of water of crystallisation in MgSO4⋅7H2O=2467×18×100=51. 2 %.
What is Product for MgSO4 and H2O?
The naturally occurring stable hydrated states in the MgSO4/H2O system are epsomite (MgSO4·7H2O), hexahydrite (MgSO4·6H2O), kieserite (MgSO4·1H2O) and products of total dehydration of high hydrates, MgSO4 anhydrous.
Is MgSO4 a hydrate?
Therefore, the value of 𝑥 must be seven, and the chemical formula of the magnesium sulfate hydrate is MgSO4⋅7H2O.
What is the Colour of MgSO4 7H2O?
This is a deep blue crystal. But when heated, the water evaporates, and the compound turns white with a faint blue color.
What is feso4 7H2O?
Iron(II) sulfate heptahydrate.
What is the name for MgSO4 * 2h2o?
Magnesium sulfateMagnesium sulfate / IUPAC ID
How many atoms are in MgSO4 7H2O?
Explanation: In MgSO4 , the number of oxygen atoms is represented by the subscript directly next to the O . In 7H2O , the coefficient 7 can be multiplied throughout each element in the molecule. There is a total of 4+7=11 oxygen atoms.
What is the formula weight of MgSO4 ∙ 7H2O?
0:051:21Molar Mass / Molecular Weight of MgSO4 · 7H2O (Magnesium Sulfate ...YouTubeStart of suggested clipEnd of suggested clipWe first need to figure out the atomic masses for each of the elements for magnesium. We have 24.31MoreWe first need to figure out the atomic masses for each of the elements for magnesium. We have 24.31 grams per moles sulfur is 32.07 oxygen 16.00 hydrogen is 1.01. And then we have oxygen it's at 16.00
What is the balanced equation for the dehydration of MgSO4 7H2O?
MgSO4*7H2O = MgSO4 + H2O - Chemical Equation Balancer.
What is the molar mass of MgSO4 * 7H2O?
246.48Magnesium SulfateProduct CodeMG4998Molecular weight246.48FormsolidAppearancewhite crystalsMolecular formulaMgSO4 · 7H2O4 more rows
What happens when MgSO4 7H2O is heated?
When magnesium sulphate heptahydrate crystals are gently heated, it loses seven water molecules and becomes anhydrous magnesium sulphate.
What is hydrate formula?
Formula of a Hydrate (Anhydrous Solid⋅xH2O) In order to determine the formula of the hydrate, [Anhydrous Solid⋅xH2O], the number of moles of water per mole of anhydrous solid (x) will be calculated by dividing the number of moles of water by the number of moles of the anhydrous solid (Equation 2.12. 6).
7.2 FDA National Drug Code Directory
MAGNESIUM SULFATE HEPTAHYDRATE is an active ingredient in 40 products including: '50% MAGNESIUM SULFATE ', 'Daily Care Epsom Salt', and 'Diarrhea Relief'.
9.1 Uses
Safer Chemical Classes -> Green circle - The chemical has been verified to be of low concern
10.1 Hazards Identification
Green circle - The chemical has been verified to be of low concern based on experimental and modeled data.
What is magnesium sulfate used for?
Magnesium sulfate for neuroprotection in the setting of chorioamnionitis.
Is magnesium sulfate a hydrate?
Magnesium Sulfate Hydrate is generally immediately available in most volumes, including bulk quantities. American Elements can produce most materials in high purity and ultra high purity (up to 99.99999%) forms and follows applicable ASTM testing standards; a range of grades are available including Mil Spec (military grade), ACS, Reagent and Technical Grade, Food, Agricultural and Pharmaceutical Grade, Optical Grade, USP and EP/BP (European Pharmacopoeia/British Pharmacopoeia). We can also produce materials to custom specifications by request, in addition to custom compositions for commercial and research applications and new proprietary technologies. Typical and custom packaging is available, as is additional research, technical and safety (MSDS) data.
What is the decimal percent of water in a hydrate?
decimal percent of water in the hydrate ---> 36.0296 g / 165.8686 g = 0.217218
How many hydrates does sodium carbonate have?
Comment: sodium carbonate forms three hydrates and the above is not one of them. This is a problem probably crafted so that you cannot look up possible answers via the InterTubez®. Just sayin'.
How much sodium carbonate does HCl react with?
This means that the HCl reacted with 0.013722 mole of sodium carbonate.
