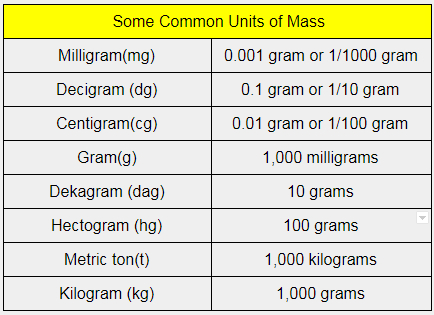
What are the basic units for mass?
Mass is defined as the amount of matter an object contains. The basic unit of mass in SI units is kilogram. Newtons second law states that the acceleration of an object is dependent upon two factors. 1) the net force acting upon the object and 2) the mass of the object.
How do you calculate mass formula?
The steps for determining a compound’s empirical formula are as follows:
- Calculate the mass of each element in grams. Element percentage = mass in grams = m
- Count the number of moles of each type of atom that is present. ...
- Divide the number of moles of each element from the smallest number of moles found in the previous step. ...
What are examples of formula units?
Formula unit helps us in giving an empirical formula that can be used for understanding the compound. It is the unit that represents an entire compound. For example, we consider NaCl compound here Na or sodium is a positive ion and Cl or chlorine is a negative ion.
What is the mathematical formula for mass?
mass=density×volume (m=ρV). Density is a measure of mass per unit of volume, so the mass of an object can be determined by multiplying density by volume. mass=force÷acceleration (m=F/a).

What is formula unit mass Class 9 Example?
The sum of atomic masses of all the atoms as present in the empirical or simple formula of a compound is called formula mass. The units of formula mass “atomic mass unit” (amu). For e.g NaCl= 23 + 35.5 = 58.5 amu.
Why do we use formula unit mass?
Statement I: Formula unit mass is used to calculate the mass of ionic compounds. Statement II: Formula unit mass is calculated by adding the atomic masses of all the ions involved in the compound.
What is unit mass in physics?
The SI unit of mass is the kilogram (kg). In science and technology, the weight of a body in a particular reference frame is defined as the force that gives the body an acceleration equal to the local acceleration of free fall in that reference frame.
What is formula unit with example?
Definition of formula unit (of an ionic compound that does not form molecules, as most salts) the chemical formula with the least number of elements out of the set of empirical formulas having the same proportion of ions as elements: NaCl is the formula unit for the ionic compound sodium chloride.
What is the use of formula unit?
In chemistry, a formula unit is the empirical formula of any ionic or covalent network solid compound used as an independent entity for stoichiometric calculations. It is the lowest whole number ratio of ions represented in an ionic compound.
What is the difference between formula unit and formula mass?
Formula mass of a substance is actually the sum of atomic masses of constituents atoms in an ionic compound or we can say that the sum of atomic masses of the ions present in the formula unit of an ionic compound.
What is the difference between formula unit mass and molecular mass?
This is because the formula mass and molecular mass are the same. The main difference is that the formula mass is calculated by adding the masses of atoms present in the simplest formula that can be given for a molecule. In contrast, the molecular mass is calculated using the number of atoms present in a molecule.
What's the difference between formula mass and molar mass?
The key difference between formula mass and molar mass is that, the formula mass of a molecule or a compound is the sum of the atomic weights of the atoms in its empirical formula while molar mass is the mass in grams of 1 mol of substance.
What is the unit of mass?
There are various units for calculating mass, like, kilograms, grams, lbs, pounds, etc., but the SI unit of mass is "kilograms" or kg. Every unit of mass can be converted to other units by using a proper conversion formula without affecting the meaning and essence of the quantity to be measured.
What is the formula used to calculate the mass of an object?
The formula used to calculate the mass of an object is, Density × Volume.
What Is Mass?
In Physics, mass is the most basic property of matter and it is one of the fundamental quantities. Mass is defined as the amount of matter present in a body. The SI unit of mass is the kilogram (kg). The formula of mass can be written as:
What is mass in science?
Mass can be best understood as the amount of matter present in any object or body. Everything we see around us has mass. For example, a table, a chair, your bed, a football, a glass, and even air has mass. That being said, all objects are light or heavy because of their mass. In this lesson, we will learn what is mass, how to calculate it, ...
How to calculate mass?
•Mass is always constant for a body. •One way to calculate mass: Mass = volume × density. •Weight is the measure of the gravitational force acting on a mass. The SI unit of mass is "kilogram".
What is the difference between density and mass?
Mass measures the amount of matter present in a substance . Density shows the amount of matter in a given space for a substance. Density and mass can be the same as unit volume.
What is mass energy?
Mass can also be defined as "stored energy in particles."
What is the sum of the atomic masses of all the atoms in a formula unit of a compound?
The sum of the atomic masses of all the atoms in a formula unit of a compound is called the formula unit mass of the compound.
How to find the mass of CaCl2?
the formula unit mass of CaCl2 = atomic mass of Ca + [ 2 x atomic mass of Cl]
What is relative formula mass?
This simply means the calculation is performed using relative atomic weight values for the elements, which are based on the natural isotopic ratio of elements found in Earth's atmosphere and crust.
How to find the mass of a gram?
Gram formula mass is calculated as: gram formula mass = mass solute / formula mass of solute. You'll usually be asked to give the gram formula mass for 1 mole of a substance.
What is the formula of a molecule?
The formula mass of a molecule (also known as formula weight) is the sum of the atomic weights of the atoms in the empirical formula of the compound. Formula weight is given in atomic mass units (amu).
How to find the relative formula of sodium oxide?
The relative atomic mass of carbon is 12 and of oxygen is 16, so the relative formula mass is: 12 + 16 = 28. To find the relative formula mass of sodium oxide, Na 2 O, you multiply the relative atomic mass of sodium times its subscript and add the value to the relative atomic mass of oxygen: (23 x 2) + 16 = 62.
What is the mass of a gram?
So, the gram formula mass is 474 g.
How many grams are in a mole of sodium oxide?
One mole of sodium oxide has a relative formula mass of 62 grams.