There are four quantum numbers:
- n - principal quantum number: describes the energy level
- ℓ - azimuthal or angular momentum quantum number: describes the subshell
- m ℓ or m - magnetic quantum number: describes the orbital of the subshell
- m s or s - spin quantum number: describes the spin
What does the fourth quantum number indicate?
The four quantum numbers used to describe the electrons are n=2, ℓ=1, m=1, 0, or -1, and s=1/2 (the electrons have parallel spins). Not Just for Electrons While quantum numbers are commonly used to describe electrons, they may be used to describe the nucleons (protons and neutrons) of an atom or elementary particles.
What are the four quantum numbers?
They are:
- PRINCIPAL QUANTUM NUMBER (n) - Represents the main energy level, or shell, occupied by an electron. ...
- SECONDARY QUANTUM NUMBER (l ) - Represents the energy sublevel, or type of orbital, occupied by the electron. ...
- MAGNETIC QUANTUM NUMBER (ml ) - Represents the number of possible orientations in 3-D space for each type of orbital. ...
What are the rules of quantum numbers?
What Are The Rules For Electron Configuration?
- Quantum Numbers. These are a set of 4 numbers that together define the position of each individual electron in the atom.
- Aufbau Principle. ...
- Hund’s Rule of Maximum Multiplicity. ...
- Pauli’s Exclusion Principle. ...
What are some examples of quantum numbers?
There are four quantum numbers:-
- Principal quantum number (n) : It defines the main shell to which an electron belongs. It generally tells the size of the orbital. ...
- Azimuthal quantum number (l) : It tells us the number of subshells present in the main shell and the orbital to which an electron belongs. ...
- Magnetic quantum number (m) : It informs the level of degenera
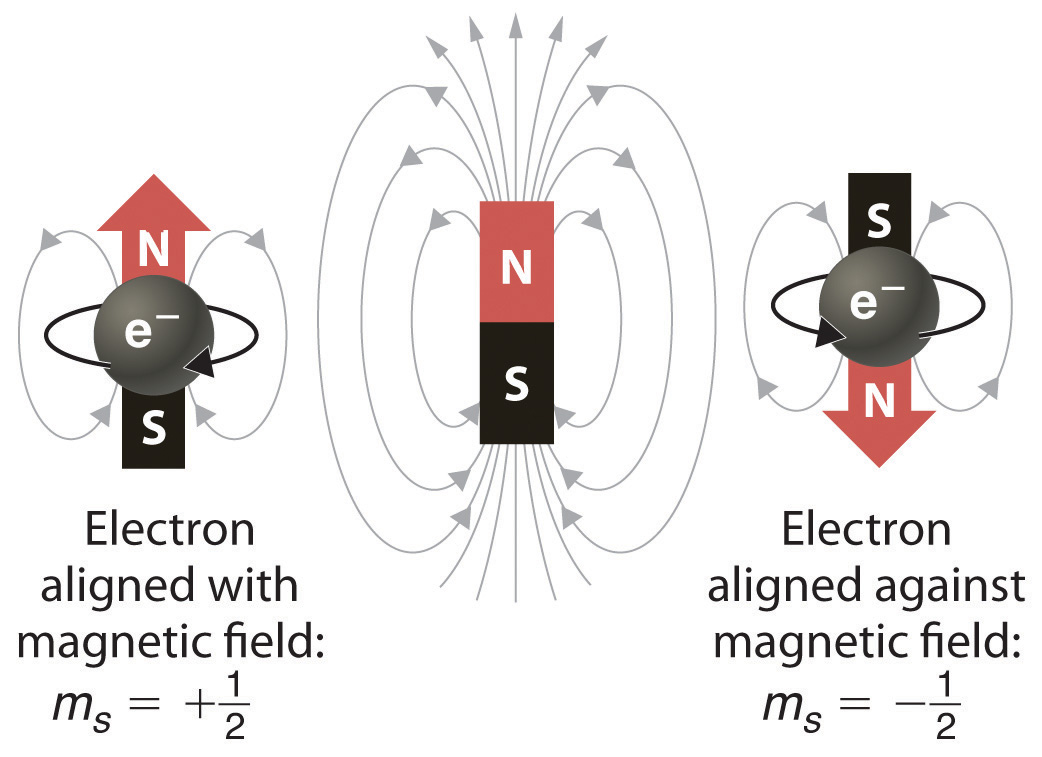
What does the 4th quantum number represent?
Each electron in an atom is described by four different quantum numbers. The first three (n, l, ml) specify the particular orbital of interest, and the fourth (ms) specifies how many electrons can occupy that orbital.
What are the 4 quantum numbers?
In atoms, there are a total of four quantum numbers: the principal quantum number (n), the orbital angular momentum quantum number (l), the magnetic quantum number (ml), and the electron spin quantum number (ms).
How do you write 4 quantum numbers?
1:464:24How To Determine The 4 Quantum Numbers From an ... - YouTubeYouTubeStart of suggested clipEnd of suggested clipWell we know n is 3 because you know that number it's in the third energy level for the d sub levelMoreWell we know n is 3 because you know that number it's in the third energy level for the d sub level we know l is two remember for acid zero for p is one for d it's two for f is three.
What are the 4 quantum numbers of 4s?
Therefore the quantum no's of the differentiating electron in 4s-orbital are; n = 4, l = 0, m = 0 and s = +1/2.
What are the 4 quantum numbers for hydrogen?
There are four quantum numbers: n, ℓ, mℓ, and ms. Each one is a particular factor in an equation describing a property of the electron.
What is the first quantum number?
The first quantum number is the principle quantum number, n. This quantum number can be 1, 2, 3... It is most closely related to the shell or orbits of the Bohr model. (A) A contour representation of an s orbital.
What are the four quantum numbers of 1s 1?
-1, 0, 1, +l. 4.
What is the total number of orbitals in n 4?
16Therefore in n=4, number of subshells=4, orbitals=16 and number of electrons =32.
What is the principal quantum number?
In quantum mechanics, the principal quantum number (symbolized n) is one of four quantum numbers assigned to each electron in an atom to describe that electron's state. Its values are natural numbers (from 1) making it a discrete variable.
What are n and L value for 4s?
For the 4s orbital, the principal quantum number n is 4 since it is at the 4th shell of main orbitals. For n = 4, we can have l as 0, 1, 2, and 3, which represent s, p,d and f orbitals. In this question, the electron is in s state, so l = 0. Thus, n = 4 and l = 0.
Which set of quantum numbers describes a 4s orbital?
The angular momentum quantum number determines the shape of the orbital, and the shape is what is reported. s orbitals are designated by ℓ=0. So a 4s orbital would be described by n=4 and ℓ=0.
What is the azimuthal quantum number of 4s?
The orbital is 4s, so the principal quantum number is 4. The azimuthal quantum number 'l' for 's' orbital is 0.
What is quantum number?
The angular quantum number (l) describes the shape of the orbital. Orbitals have shapes that are best described as spherical (l = 0), polar (l = 1), or cloverleaf (l = 2). They can even take on more complex shapes as the value of the angular quantum number becomes larger.
What do the four quantum numbers describe about an electron 1 point?
Quantum numbers are values that describe the energy or energetic state of an atom's electron. The numbers indicate an electron's spin, energy, magnetic moment and angular moment.
What are quantum numbers in organic chemistry?
The quantum numbers are parameters that describe the distribution of electrons in the atom, and therefore its fundamental nature. They are: 1. PRINCIPAL QUANTUM NUMBER (n) - Represents the main energy level, or shell, occupied by an electron.
What are quantum numbers and their significance?
Quantum numbers is defined as the set of numbers that used to describe the position and energy of the electron in an atom. There are four quantum numbers, namely, principal, azimuthal, magnetic and spin quantum numbers. Principal Quantum Number (n): signifies the size of the electron cloud.
What are the four quantum numbers?
The four quantum numbers are the principle quantum number, n , the angular momentum quantum number, l, the magnetic quantum number, ml , and the electron spin quantum number, ms.
Which quantum number describes an electron?
Therefore, n describes the shell, both n and l describe a subshell, n, l, and ml describe an orbital, and all four quantum numbers ( n, l, ml, ms) describe an electron.
What is the angular momentum quantum number?
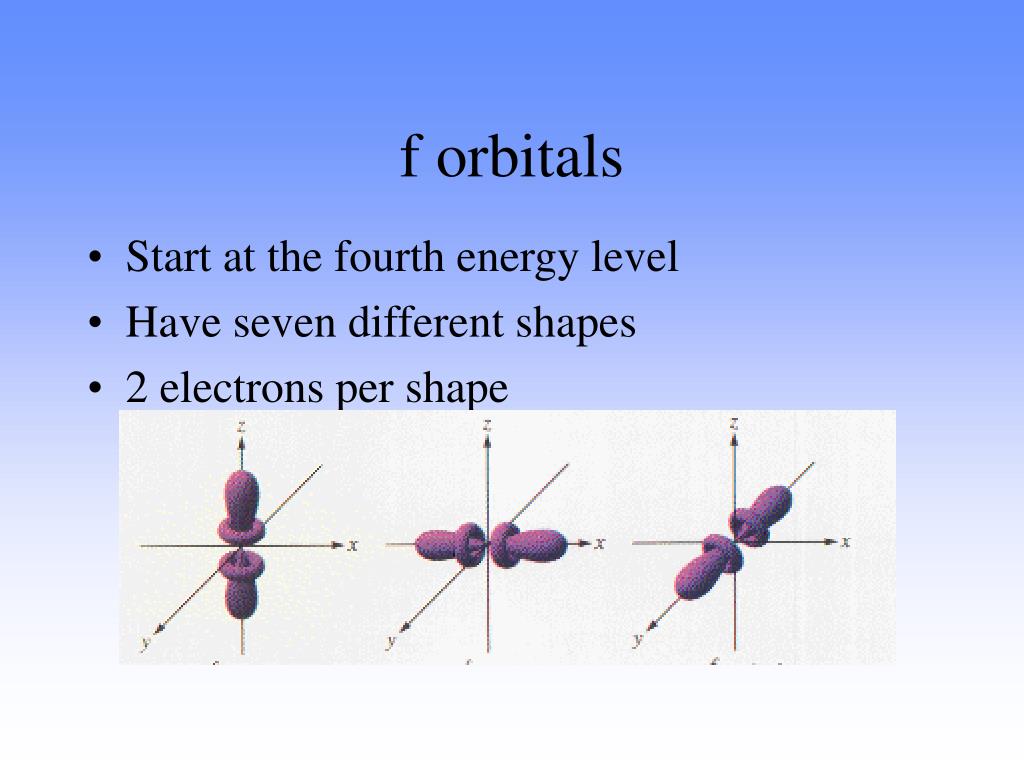
The angular momentum quantum number , l, describes the shape of the subshell and its orbitals, where l = 0,1,2,3... corresponds to s,p,d, and f subshells (containing s,p,d,f orbitals), respectively .
What is the magnetic quantum number?
The magnetic quantum number , ml, describes the orientation of the orbitals (within the subshells) in space. The possible values for ml of any type of orbital ( s,p,d,f ...) is given by any integer value from −l to l.
How many electrons are in an orbital?
Remember that there are only two electrons to every orbital, and that they should have opposite spins (think Pauli exclusion principle). This tells us that there are two electrons per orbital, or per ml value, one with an ms value of + 1 2 and one with an ms value of − 1 2.
How many possible orientations does a 2p orbital have?
So, for a 2p orbital with n = 2 and l = 1, we can have m1 = − 1,0,1. This tells us that the p orbital has 3 possible orientations in space.
